Bromine »
PDB 101d-1e5a »
1dg7 »
Bromine in PDB 1dg7: Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210
Enzymatic activity of Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210
All present enzymatic activity of Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210:
1.5.1.3;
1.5.1.3;
Protein crystallography data
The structure of Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210, PDB code: 1dg7
was solved by
R.Li,
R.Sirawaraporn,
P.Chitnumsub,
W.Sirawaraporn,
J.Wooden,
F.Athappilly,
S.Turley,
W.G.Hol,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.80 |
| Space group | P 41 |
| Cell size a, b, c (Å), α, β, γ (°) | 60.630, 60.630, 59.130, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.8 / 24.6 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210
(pdb code 1dg7). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total only one binding site of Bromine was determined in the Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210, PDB code: 1dg7:
In total only one binding site of Bromine was determined in the Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210, PDB code: 1dg7:
Bromine binding site 1 out of 1 in 1dg7
Go back to
Bromine binding site 1 out
of 1 in the Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210
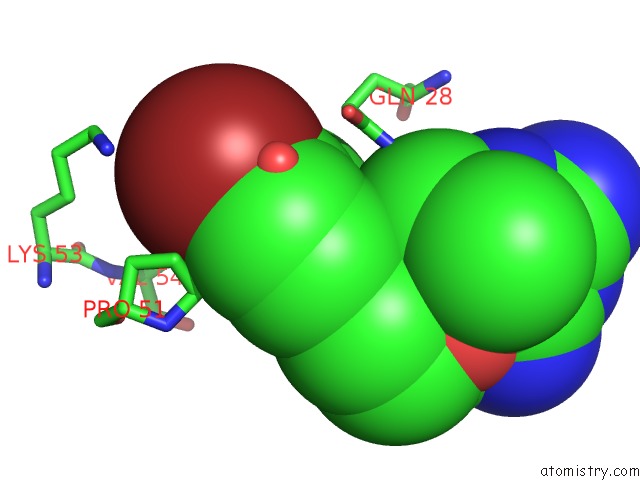
Mono view
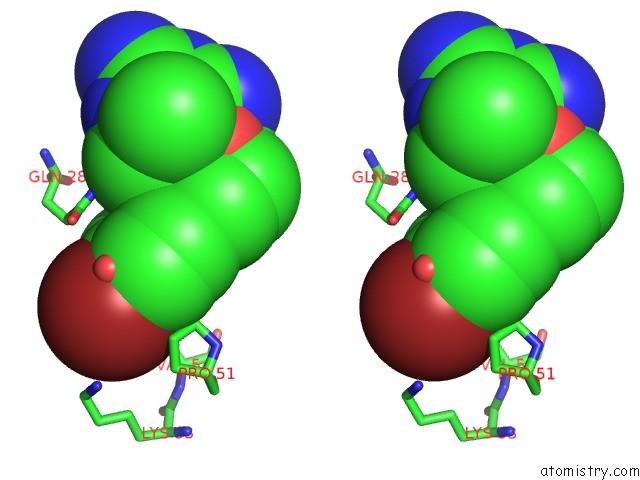
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Dihydrofolate Reductase of Mycobacterium Tuberculosis Complexed with Nadph and 4-Bromo WR99210 within 5.0Å range:
|
Reference:
R.Li,
R.Sirawaraporn,
P.Chitnumsub,
W.Sirawaraporn,
J.Wooden,
F.Athappilly,
S.Turley,
W.G.Hol.
Three-Dimensional Structure of M. Tuberculosis Dihydrofolate Reductase Reveals Opportunities For the Design of Novel Tuberculosis Drugs. J.Mol.Biol. V. 295 307 2000.
ISSN: ISSN 0022-2836
PubMed: 10623528
DOI: 10.1006/JMBI.1999.3328
Page generated: Mon Jul 7 03:05:17 2025
ISSN: ISSN 0022-2836
PubMed: 10623528
DOI: 10.1006/JMBI.1999.3328
Last articles
Cl in 5PA7Cl in 5PA6
Cl in 5PA5
Cl in 5PA3
Cl in 5P9Z
Cl in 5PA0
Cl in 5PA1
Cl in 5PA2
Cl in 5P9Y
Cl in 5P9X