Bromine »
PDB 1iha-1m9r »
1m9r »
Bromine in PDB 1m9r: Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
Enzymatic activity of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
All present enzymatic activity of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound:
1.14.13.39;
1.14.13.39;
Protein crystallography data
The structure of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound, PDB code: 1m9r
was solved by
R.J.Rosenfeld,
E.D.Garcin,
K.Panda,
G.Andersson,
A.Aberg,
A.V.Wallace,
D.J.Stuehr,
J.A.Tainer,
E.D.Getzoff,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.65 / 2.56 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 68.625, 90.280, 155.490, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.8 / 26.6 |
Other elements in 1m9r:
The structure of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound also contains other interesting chemical elements:
| Iron | (Fe) | 2 atoms |
| Zinc | (Zn) | 1 atom |
Bromine Binding Sites:
The binding sites of Bromine atom in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
(pdb code 1m9r). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 4 binding sites of Bromine where determined in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound, PDB code: 1m9r:
Jump to Bromine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Bromine where determined in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound, PDB code: 1m9r:
Jump to Bromine binding site number: 1; 2; 3; 4;
Bromine binding site 1 out of 4 in 1m9r
Go back to
Bromine binding site 1 out
of 4 in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
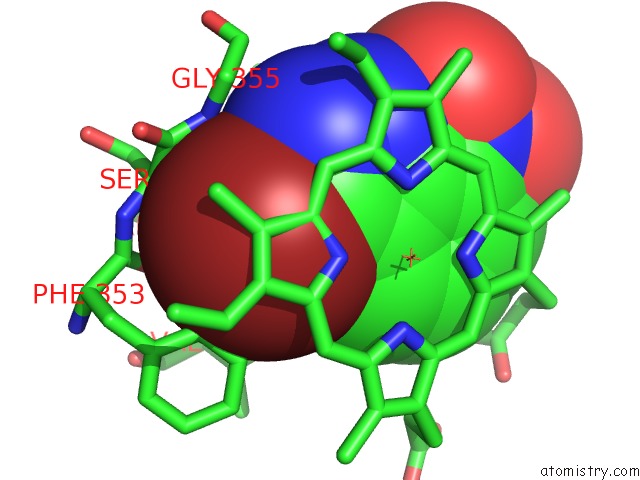
Mono view
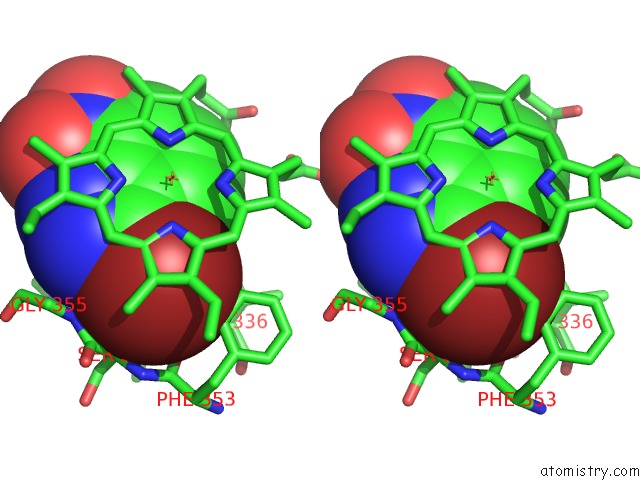
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound within 5.0Å range:
|
Bromine binding site 2 out of 4 in 1m9r
Go back to
Bromine binding site 2 out
of 4 in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
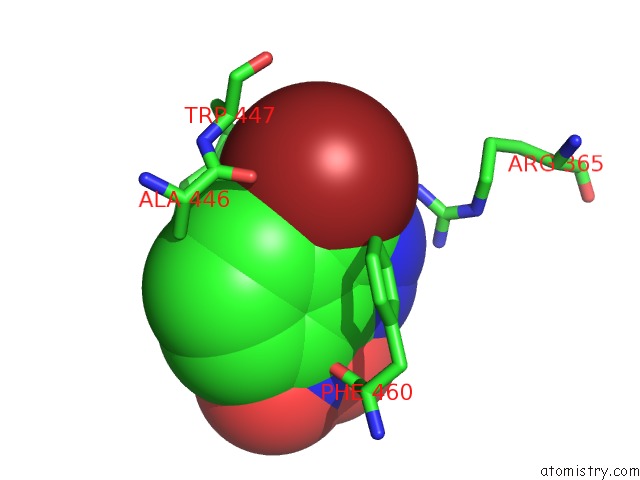
Mono view
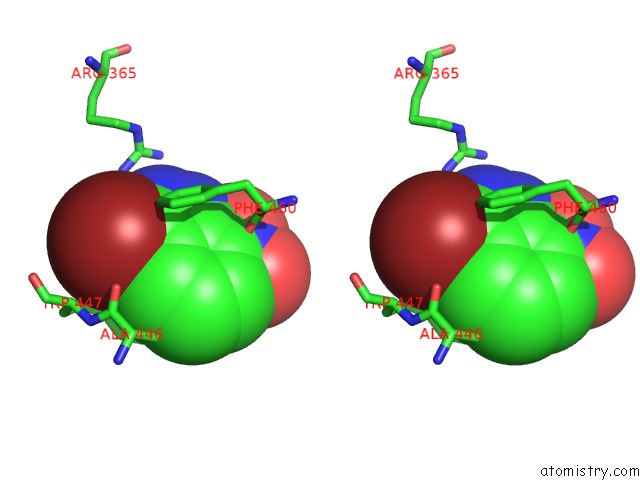
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound within 5.0Å range:
|
Bromine binding site 3 out of 4 in 1m9r
Go back to
Bromine binding site 3 out
of 4 in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
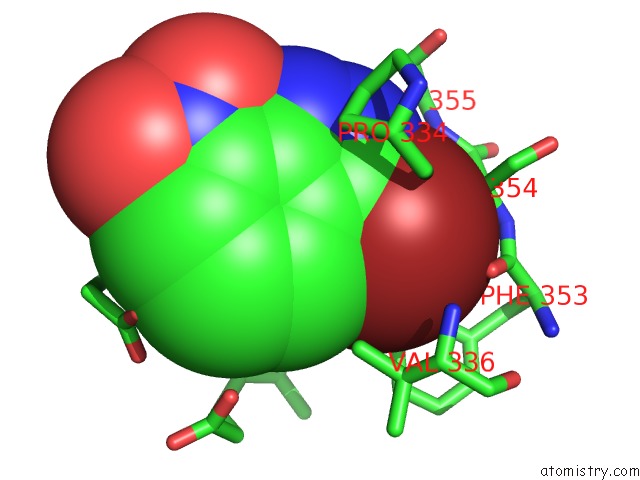
Mono view
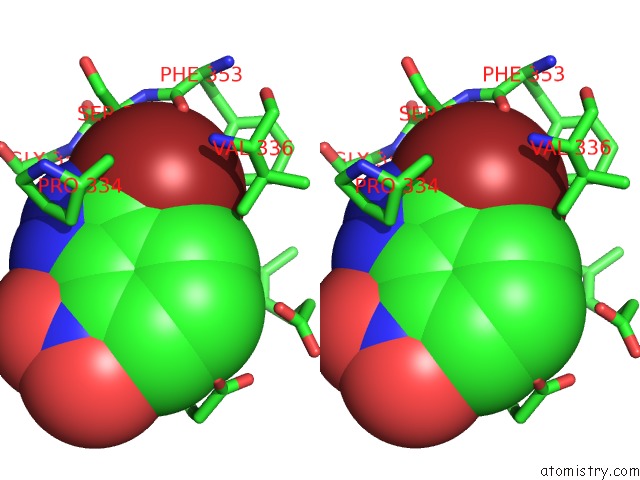
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound within 5.0Å range:
|
Bromine binding site 4 out of 4 in 1m9r
Go back to
Bromine binding site 4 out
of 4 in the Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound
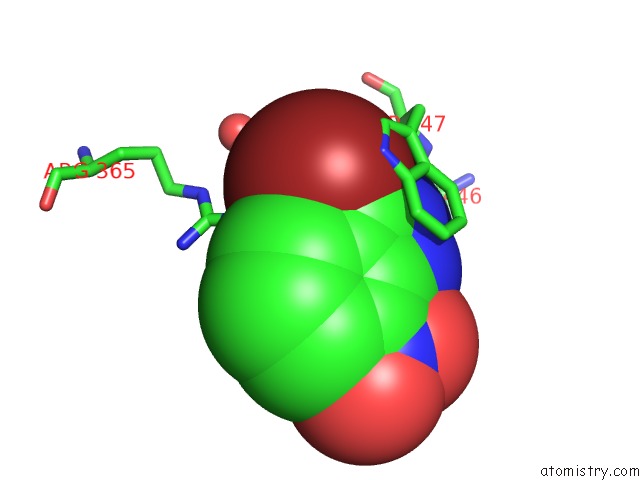
Mono view
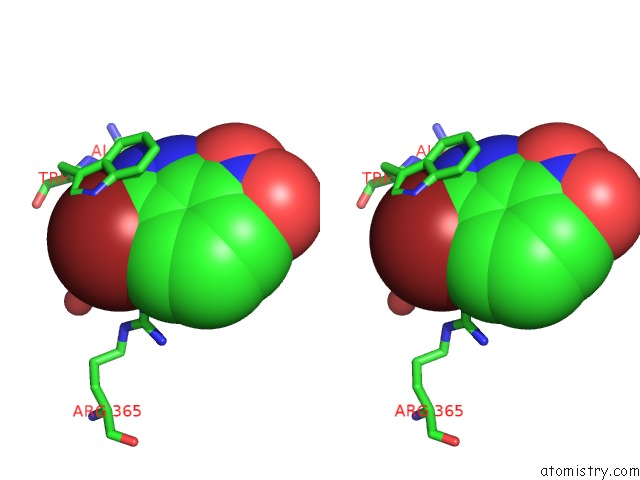
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 4 of Human Endothelial Nitric Oxide Synthase with 3-Bromo-7- Nitroindazole Bound within 5.0Å range:
|
Reference:
R.J.Rosenfeld,
E.D.Garcin,
K.Panda,
G.Andersson,
A.Aberg,
A.V.Wallace,
G.M.Morris,
A.J.Olson,
D.J.Stuehr,
J.A.Tainer,
E.D.Getzoff.
Conformational Changes in Nitric Oxide Synthases Induced By Chlorzoxazone and Nitroindazoles: Crystallographic and Computational Analyses of Inhibitor Potency Biochemistry V. 41 13915 2002.
ISSN: ISSN 0006-2960
PubMed: 12437348
DOI: 10.1021/BI026313J
Page generated: Mon Jul 7 03:26:43 2025
ISSN: ISSN 0006-2960
PubMed: 12437348
DOI: 10.1021/BI026313J
Last articles
F in 7JR6F in 7JPW
F in 7JPL
F in 7JPK
F in 7JL3
F in 7JN3
F in 7JMQ
F in 7JL2
F in 7JLR
F in 7JLM