Bromine »
PDB 1m9t-1p1y »
1o27 »
Bromine in PDB 1o27: Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution
Protein crystallography data
The structure of Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution, PDB code: 1o27
was solved by
I.I.Mathews,
A.M.Deacon,
J.M.Canaves,
D.Mcmullan,
S.A.Lesley,
S.Agarwalla,
P.Kuhn,
Joint Center For Structural Genomics (Jcsg),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.30 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 54.349, 116.428, 140.557, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.8 / 23 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution
(pdb code 1o27). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 4 binding sites of Bromine where determined in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution, PDB code: 1o27:
Jump to Bromine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Bromine where determined in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution, PDB code: 1o27:
Jump to Bromine binding site number: 1; 2; 3; 4;
Bromine binding site 1 out of 4 in 1o27
Go back to
Bromine binding site 1 out
of 4 in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution
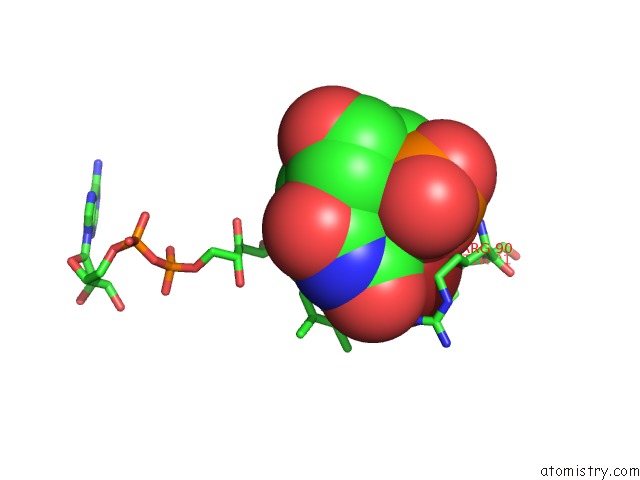
Mono view
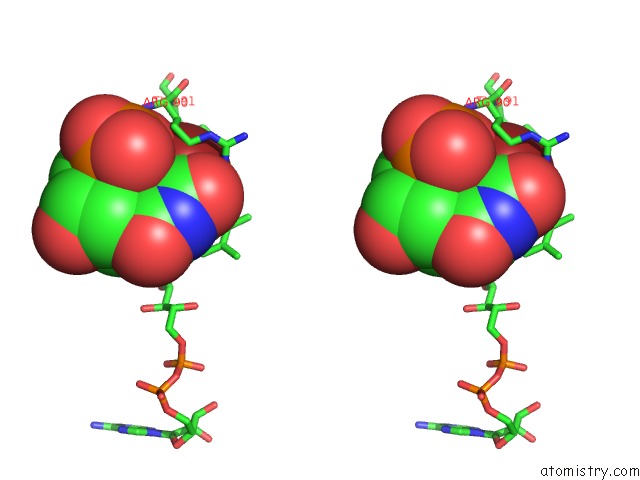
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution within 5.0Å range:
|
Bromine binding site 2 out of 4 in 1o27
Go back to
Bromine binding site 2 out
of 4 in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution
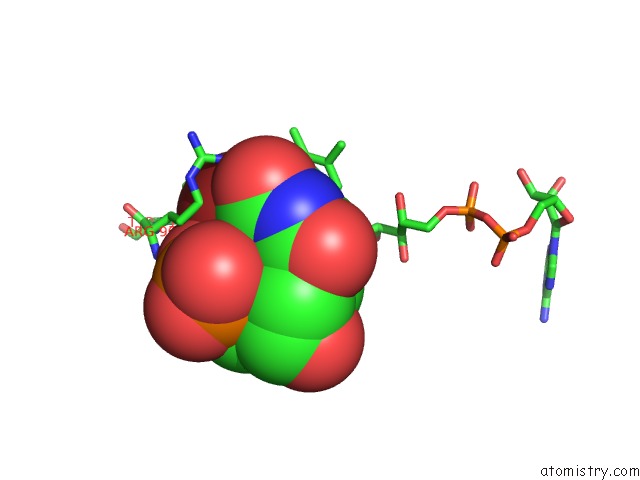
Mono view
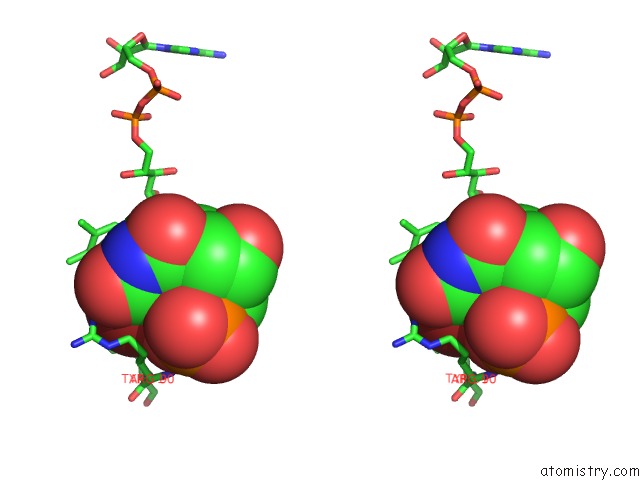
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution within 5.0Å range:
|
Bromine binding site 3 out of 4 in 1o27
Go back to
Bromine binding site 3 out
of 4 in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution
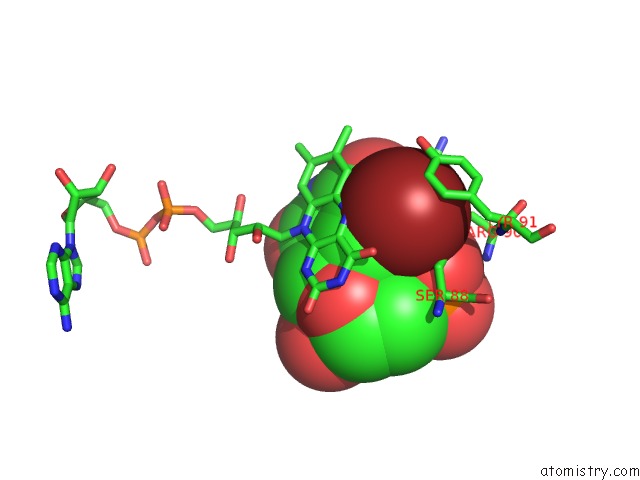
Mono view
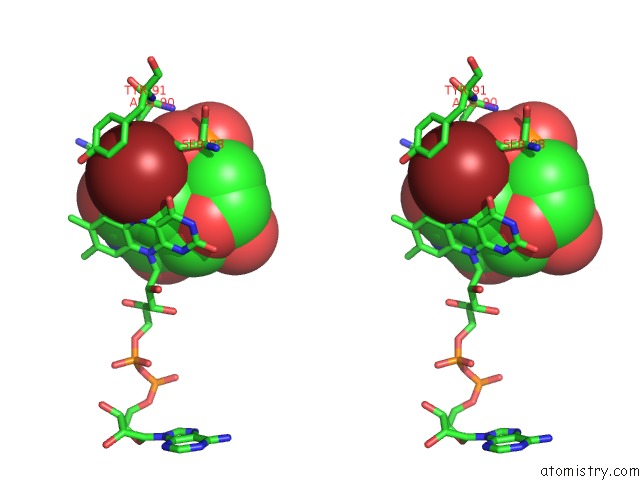
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution within 5.0Å range:
|
Bromine binding site 4 out of 4 in 1o27
Go back to
Bromine binding site 4 out
of 4 in the Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution
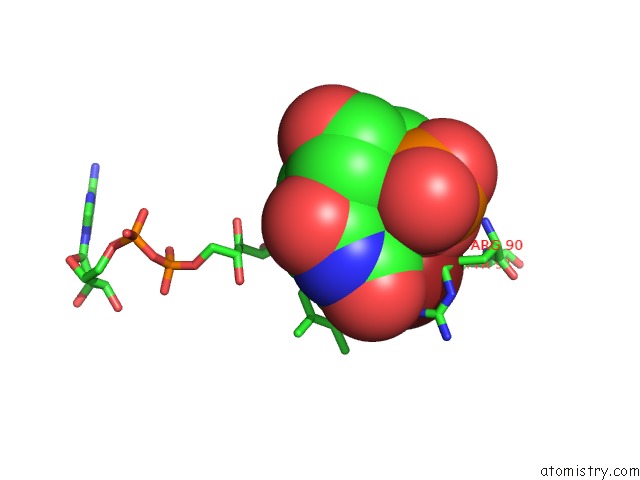
Mono view
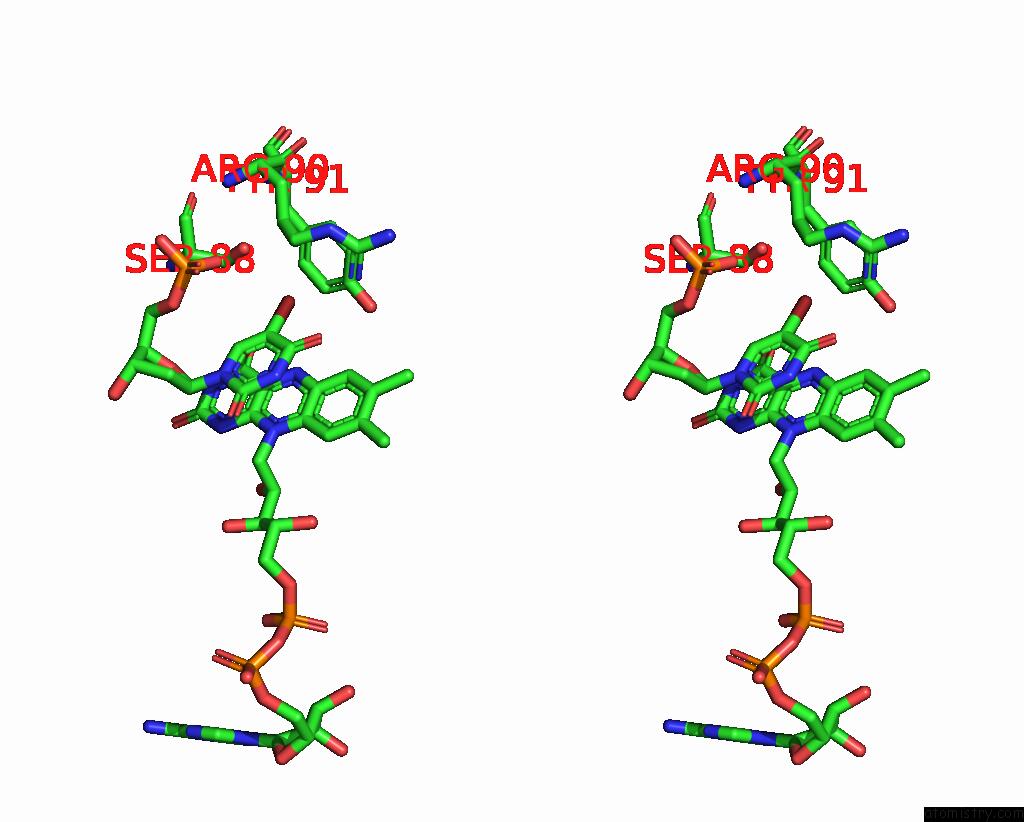
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 4 of Crystal Structure of Thymidylate Synthase Complementing Protein (TM0449) From Thermotoga Maritima with Fad and Brdump at 2.3 A Resolution within 5.0Å range:
|
Reference:
I.I.Mathews,
A.M.Deacon,
J.M.Canaves,
D.Mcmullan,
S.A.Lesley,
S.Agarwalla,
P.Kuhn.
Functional Analysis of Substrate and Cofactor Complex Structures of A Thymidylate Synthase-Complementing Protein Structure V. 11 677 2003.
ISSN: ISSN 0969-2126
PubMed: 12791256
DOI: 10.1016/S0969-2126(03)00097-2
Page generated: Mon Jul 7 03:34:06 2025
ISSN: ISSN 0969-2126
PubMed: 12791256
DOI: 10.1016/S0969-2126(03)00097-2
Last articles
Cl in 3LZ0Cl in 3LUB
Cl in 3LXT
Cl in 3LXV
Cl in 3LWF
Cl in 3LXQ
Cl in 3LX4
Cl in 3LVP
Cl in 3LW1
Cl in 3LVY