Bromine »
PDB 2qc6-2vo5 »
2qf6 »
Bromine in PDB 2qf6: HSP90 Complexed with A56322
Protein crystallography data
The structure of HSP90 Complexed with A56322, PDB code: 2qf6
was solved by
C.H.Park,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.93 / 3.10 |
| Space group | P 43 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 118.824, 118.824, 180.779, 90.00, 90.00, 90.00 |
| R / Rfree (%) | n/a / n/a |
Bromine Binding Sites:
The binding sites of Bromine atom in the HSP90 Complexed with A56322
(pdb code 2qf6). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 4 binding sites of Bromine where determined in the HSP90 Complexed with A56322, PDB code: 2qf6:
Jump to Bromine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Bromine where determined in the HSP90 Complexed with A56322, PDB code: 2qf6:
Jump to Bromine binding site number: 1; 2; 3; 4;
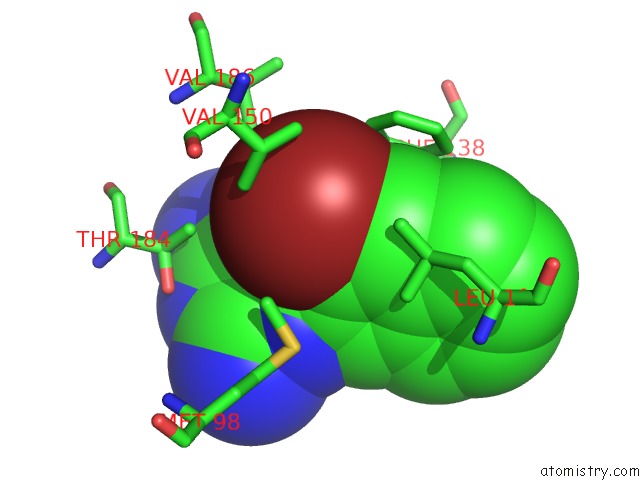
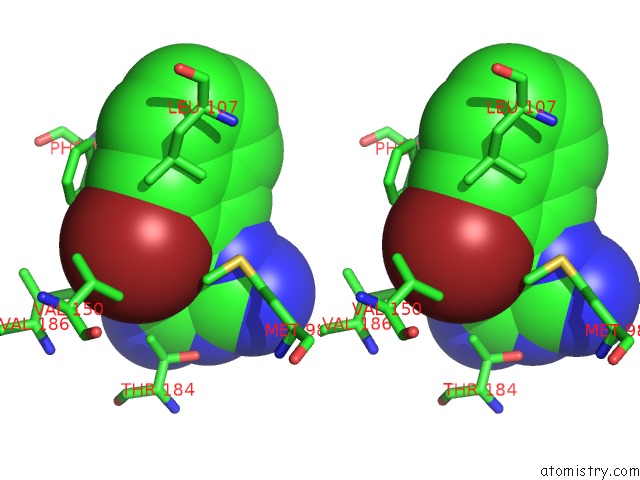
Bromine binding site 1 out of 4 in 2qf6
Go back to
Bromine binding site 1 out
of 4 in the HSP90 Complexed with A56322
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of HSP90 Complexed with A56322 within 5.0Å range:
|
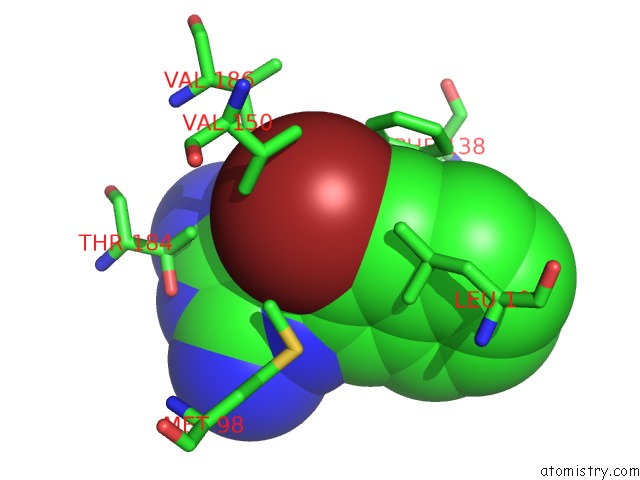
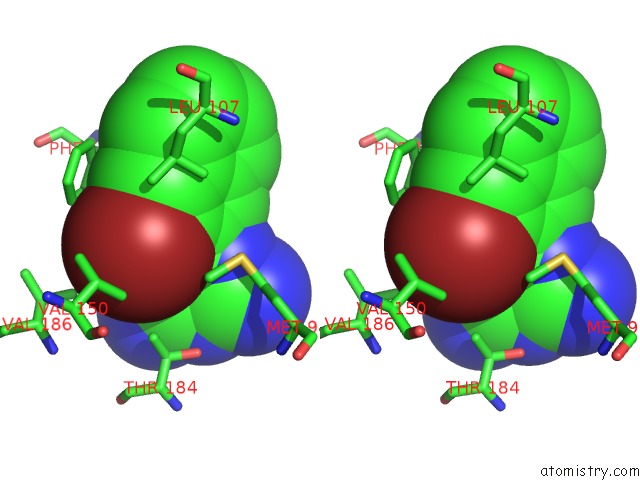
Bromine binding site 2 out of 4 in 2qf6
Go back to
Bromine binding site 2 out
of 4 in the HSP90 Complexed with A56322
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of HSP90 Complexed with A56322 within 5.0Å range:
|
Bromine binding site 3 out of 4 in 2qf6
Go back to
Bromine binding site 3 out
of 4 in the HSP90 Complexed with A56322
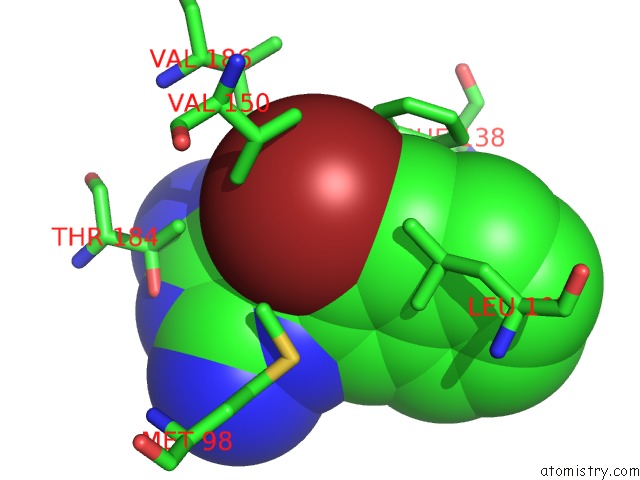
Mono view
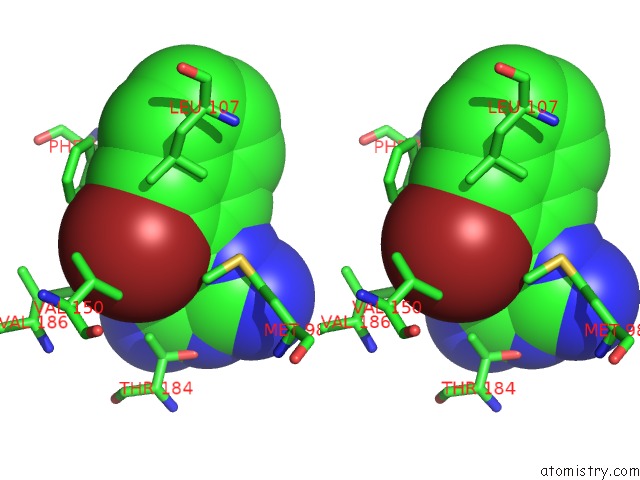
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of HSP90 Complexed with A56322 within 5.0Å range:
|
Bromine binding site 4 out of 4 in 2qf6
Go back to
Bromine binding site 4 out
of 4 in the HSP90 Complexed with A56322
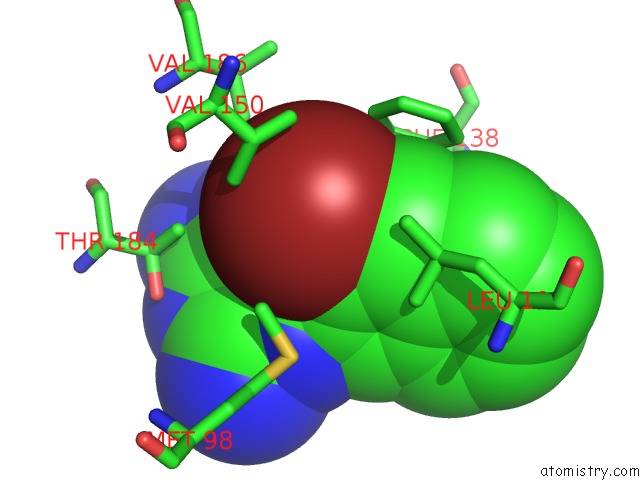
Mono view
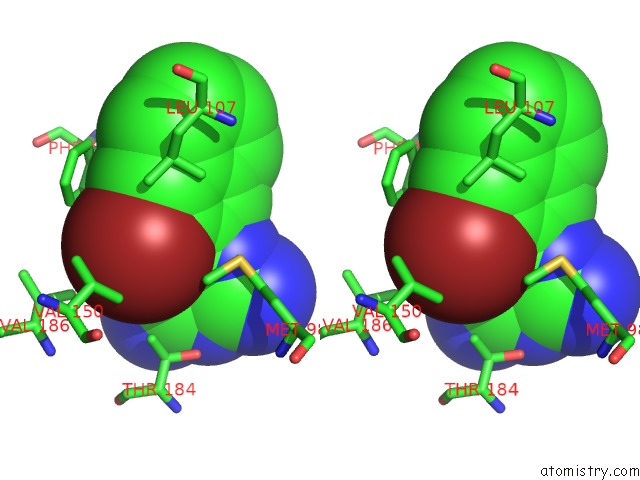
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 4 of HSP90 Complexed with A56322 within 5.0Å range:
|
Reference:
J.R.Huth,
C.Park,
A.M.Petros,
A.R.Kunzer,
M.D.Wendt,
X.Wang,
C.L.Lynch,
J.C.Mack,
K.M.Swift,
R.A.Judge,
J.Chen,
P.L.Richardson,
S.Jin,
S.K.Tahir,
E.D.Matayoshi,
S.A.Dorwin,
U.S.Ladror,
J.M.Severin,
K.A.Walter,
D.M.Bartley,
S.W.Fesik,
S.W.Elmore,
P.J.Hajduk.
Discovery and Design of Novel HSP90 Inhibitors Using Multiple Fragment-Based Design Strategies. Chem.Biol.Drug Des. V. 70 1 2007.
ISSN: ISSN 1747-0277
PubMed: 17630989
DOI: 10.1111/J.1747-0285.2007.00535.X
Page generated: Mon Jul 7 04:35:07 2025
ISSN: ISSN 1747-0277
PubMed: 17630989
DOI: 10.1111/J.1747-0285.2007.00535.X
Last articles
F in 5EIRF in 5EIW
F in 5EI3
F in 5EHE
F in 5EDG
F in 5EFH
F in 5EFQ
F in 5EDS
F in 5E4F
F in 5EAU