Bromine »
PDB 3u2d-3wk9 »
3vbx »
Bromine in PDB 3vbx: Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design
Enzymatic activity of Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design
All present enzymatic activity of Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design:
2.7.11.1;
2.7.11.1;
Protein crystallography data
The structure of Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design, PDB code: 3vbx
was solved by
J.Liu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 84.00 / 2.03 |
| Space group | P 65 |
| Cell size a, b, c (Å), α, β, γ (°) | 97.000, 97.000, 81.000, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 21.8 / 25.8 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design
(pdb code 3vbx). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total only one binding site of Bromine was determined in the Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design, PDB code: 3vbx:
In total only one binding site of Bromine was determined in the Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design, PDB code: 3vbx:
Bromine binding site 1 out of 1 in 3vbx
Go back to
Bromine binding site 1 out
of 1 in the Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design
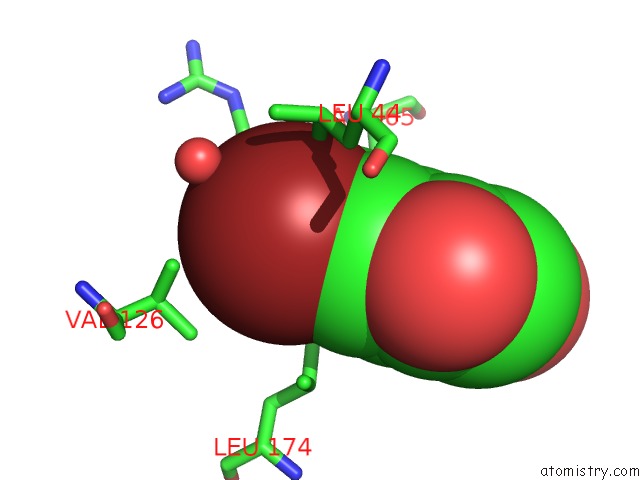
Mono view
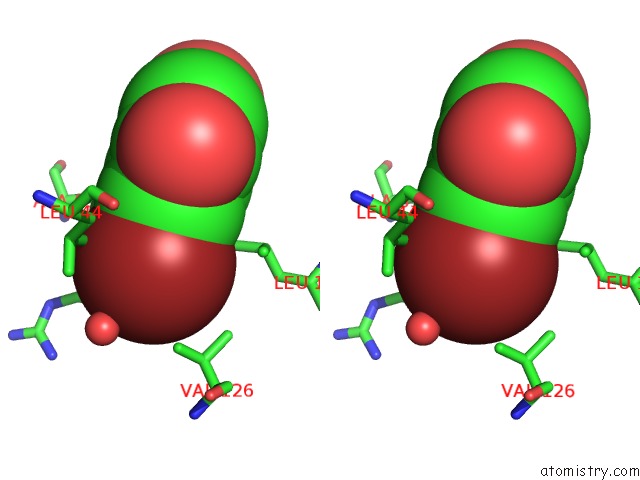
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Exploitation of Hydrogen Bonding Constraints and Flat Hydrophobic Energy Landscapes in Pim-1 Kinase Needle Screening and Inhibitor Design within 5.0Å range:
|
Reference:
A.C.Good,
J.Liu,
B.Hirth,
G.Asmussen,
Y.Xiang,
H.P.Biemann,
K.A.Bishop,
T.Fremgen,
M.Fitzgerald,
T.Gladysheva,
A.Jain,
K.Jancsics,
M.Metz,
A.Papoulis,
R.Skerlj,
J.D.Stepp,
R.R.Wei.
Implications of Promiscuous Pim-1 Kinase Fragment Inhibitor Hydrophobic Interactions For Fragment-Based Drug Design. J.Med.Chem. V. 55 2641 2012.
ISSN: ISSN 0022-2623
PubMed: 22339127
DOI: 10.1021/JM2014698
Page generated: Mon Jul 7 06:07:16 2025
ISSN: ISSN 0022-2623
PubMed: 22339127
DOI: 10.1021/JM2014698
Last articles
Cl in 5QGZCl in 5QGY
Cl in 5QGX
Cl in 5QFZ
Cl in 5QGV
Cl in 5QGS
Cl in 5QFJ
Cl in 5QF2
Cl in 5QF7
Cl in 5QED