Bromine »
PDB 4kvh-4my6 »
4kwp »
Bromine in PDB 4kwp: Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
Enzymatic activity of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
All present enzymatic activity of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution:
2.7.11.1;
2.7.11.1;
Protein crystallography data
The structure of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution, PDB code: 4kwp
was solved by
A.Ranchio,
G.Lolli,
R.Battistutta,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.24 / 1.25 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 58.448, 45.821, 63.486, 90.00, 111.15, 90.00 |
| R / Rfree (%) | 13.6 / 17 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
(pdb code 4kwp). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 4 binding sites of Bromine where determined in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution, PDB code: 4kwp:
Jump to Bromine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Bromine where determined in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution, PDB code: 4kwp:
Jump to Bromine binding site number: 1; 2; 3; 4;
Bromine binding site 1 out of 4 in 4kwp
Go back to
Bromine binding site 1 out
of 4 in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
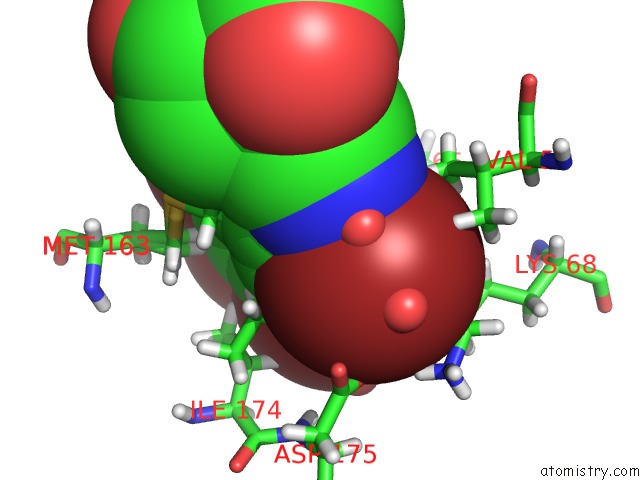
Mono view
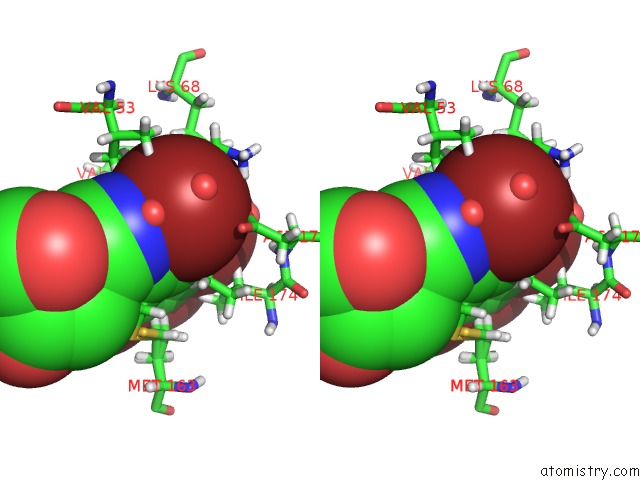
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution within 5.0Å range:
|
Bromine binding site 2 out of 4 in 4kwp
Go back to
Bromine binding site 2 out
of 4 in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
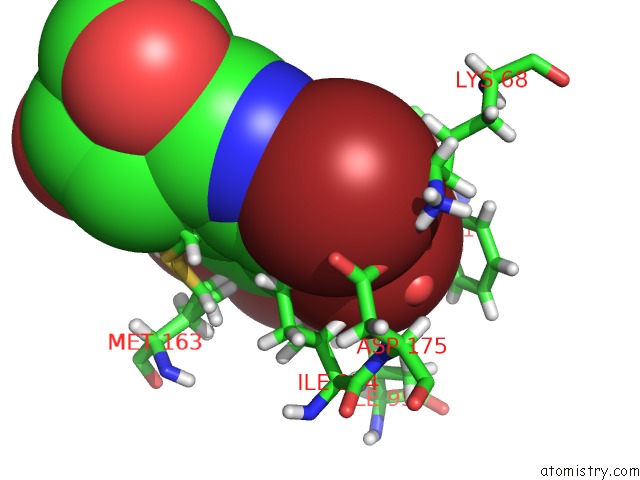
Mono view
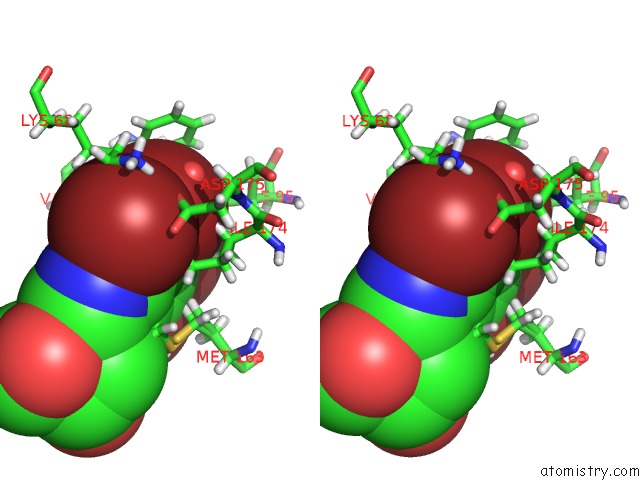
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution within 5.0Å range:
|
Bromine binding site 3 out of 4 in 4kwp
Go back to
Bromine binding site 3 out
of 4 in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
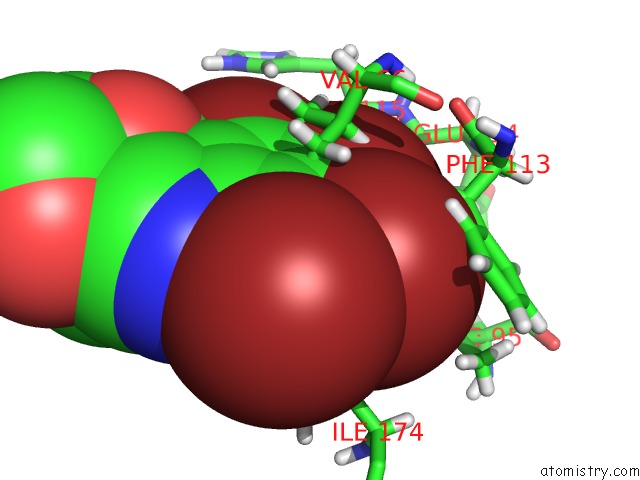
Mono view
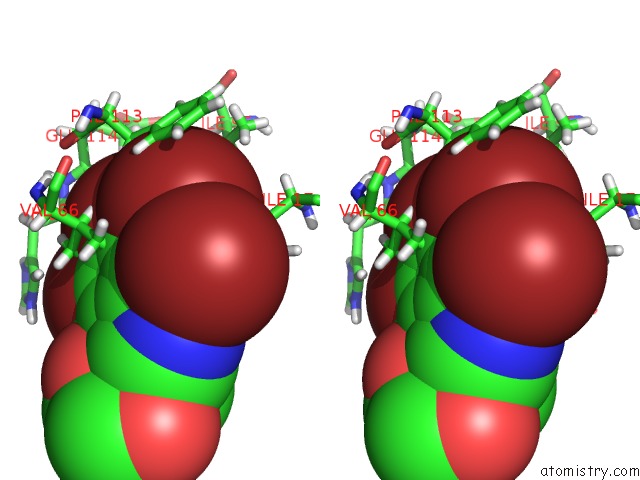
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution within 5.0Å range:
|
Bromine binding site 4 out of 4 in 4kwp
Go back to
Bromine binding site 4 out
of 4 in the Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution
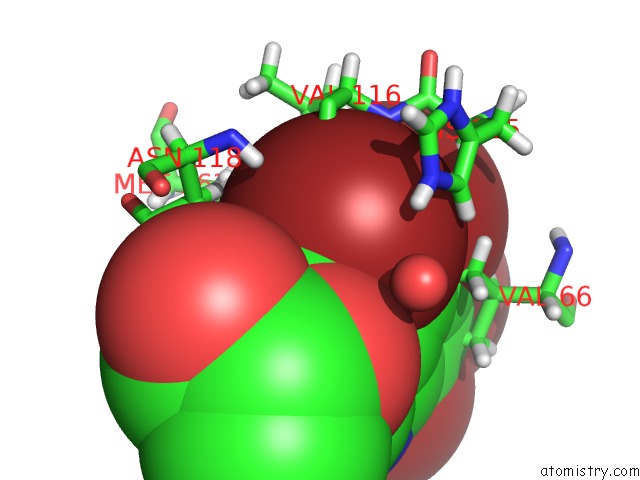
Mono view
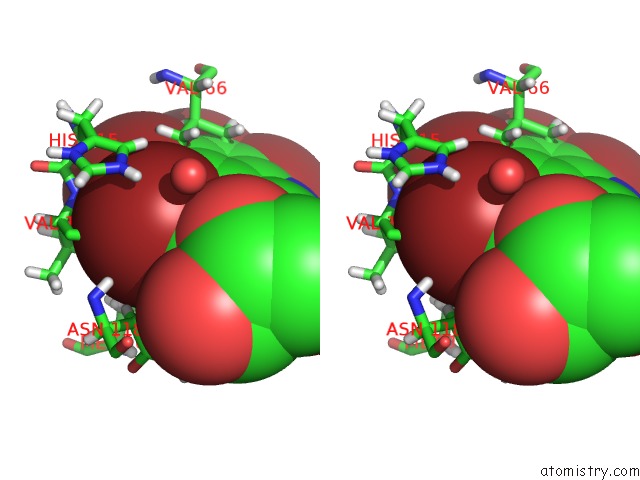
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 4 of Crystal Structure of Human CK2-Alpha in Complex with A Benzimidazole Inhibitor (K164) at 1.25 A Resolution within 5.0Å range:
|
Reference:
G.Cozza,
C.Girardi,
A.Ranchio,
G.Lolli,
S.Sarno,
A.Orzeszko,
Z.Kazimierczuk,
R.Battistutta,
M.Ruzzene,
L.A.Pinna.
Cell-Permeable Dual Inhibitors of Protein Kinases CK2 and Pim-1: Structural Features and Pharmacological Potential. Cell.Mol.Life Sci. V. 71 3173 2014.
ISSN: ISSN 1420-682X
PubMed: 24442476
DOI: 10.1007/S00018-013-1552-5
Page generated: Mon Jul 7 07:00:15 2025
ISSN: ISSN 1420-682X
PubMed: 24442476
DOI: 10.1007/S00018-013-1552-5
Last articles
Br in 9ESUBr in 9ESO
Br in 9EST
Br in 9ESP
Br in 9ESJ
Br in 9ESN
Br in 9ESL
Br in 9ESK
Br in 9EFF
Br in 9ES9