Bromine »
PDB 4kvh-4my6 »
4mk1 »
Bromine in PDB 4mk1: 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
Protein crystallography data
The structure of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease, PDB code: 4mk1
was solved by
J.D.Bauman,
D.Patel,
K.Das,
E.Arnold,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.32 / 1.85 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.932, 101.655, 66.212, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.6 / 19.8 |
Other elements in 4mk1:
The structure of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease also contains other interesting chemical elements:
| Manganese | (Mn) | 4 atoms |
Bromine Binding Sites:
The binding sites of Bromine atom in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
(pdb code 4mk1). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 3 binding sites of Bromine where determined in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease, PDB code: 4mk1:
Jump to Bromine binding site number: 1; 2; 3;
In total 3 binding sites of Bromine where determined in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease, PDB code: 4mk1:
Jump to Bromine binding site number: 1; 2; 3;
Bromine binding site 1 out of 3 in 4mk1
Go back to
Bromine binding site 1 out
of 3 in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
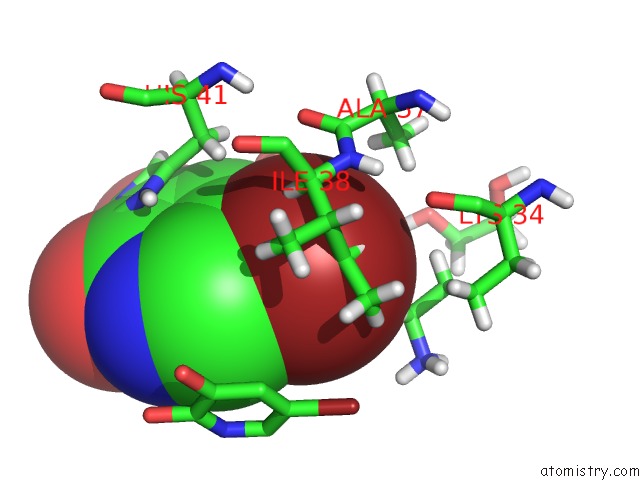
Mono view
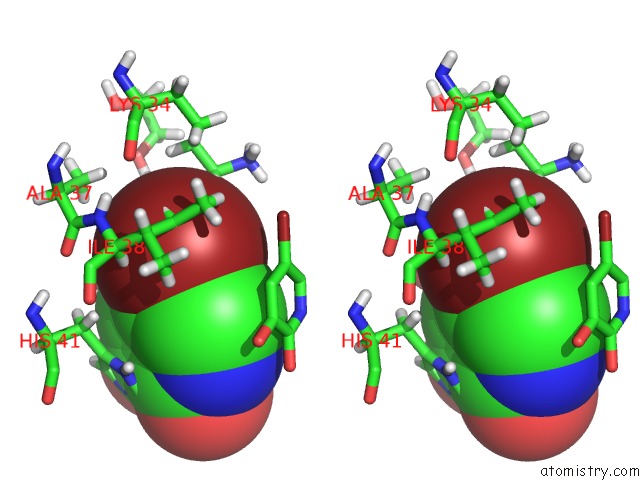
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease within 5.0Å range:
|
Bromine binding site 2 out of 3 in 4mk1
Go back to
Bromine binding site 2 out
of 3 in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
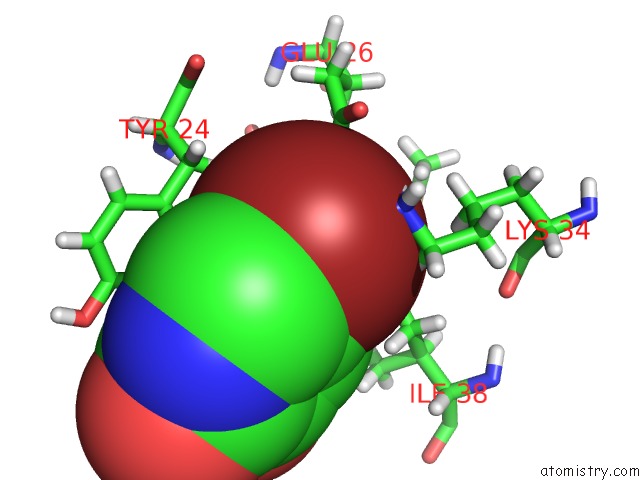
Mono view
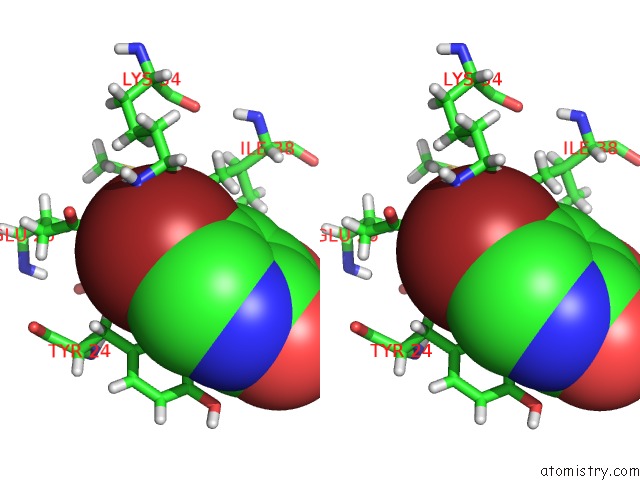
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease within 5.0Å range:
|
Bromine binding site 3 out of 3 in 4mk1
Go back to
Bromine binding site 3 out
of 3 in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
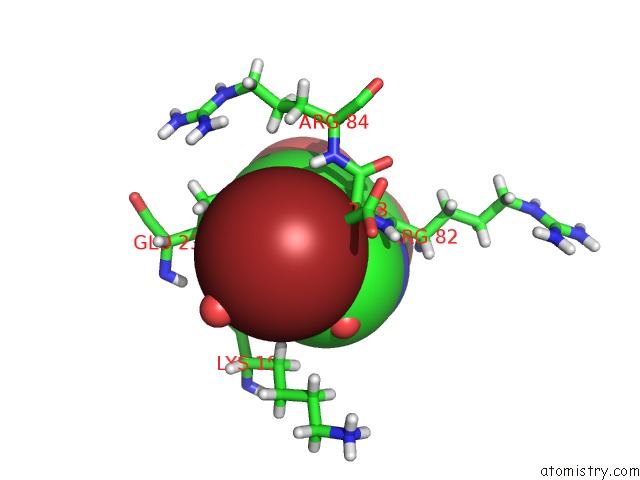
Mono view
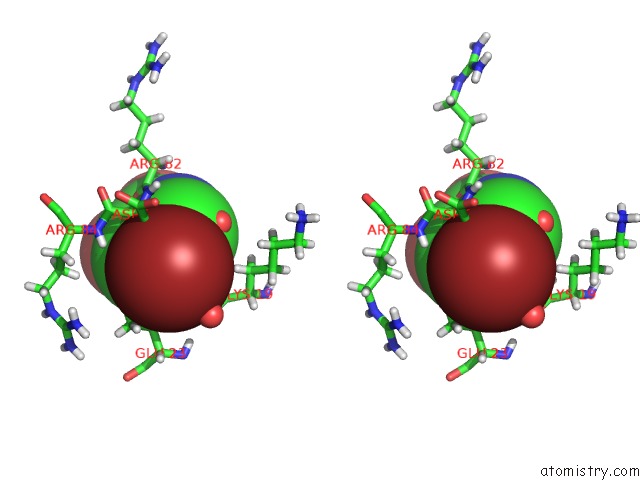
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease within 5.0Å range:
|
Reference:
J.D.Bauman,
D.Patel,
S.F.Baker,
R.S.Vijayan,
A.Xiang,
A.K.Parhi,
L.Martinez-Sobrido,
E.J.Lavoie,
K.Das,
E.Arnold.
Crystallographic Fragment Screening and Structure-Based Optimization Yields A New Class of Influenza Endonuclease Inhibitors. Acs Chem.Biol. V. 8 2501 2013.
ISSN: ISSN 1554-8929
PubMed: 23978130
DOI: 10.1021/CB400400J
Page generated: Mon Jul 7 07:06:18 2025
ISSN: ISSN 1554-8929
PubMed: 23978130
DOI: 10.1021/CB400400J
Last articles
Cl in 3M6YCl in 3M98
Cl in 3M96
Cl in 3M92
Cl in 3M81
Cl in 3M88
Cl in 3M86
Cl in 3M5V
Cl in 3M6Z
Cl in 3M6X