Bromine »
PDB 5l4h-5mqe »
5liy »
Bromine in PDB 5liy: Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
Protein crystallography data
The structure of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204, PDB code: 5liy
was solved by
A.Cousido-Siah,
F.X.Ruiz,
A.Mitschler,
J.Fanfrlik,
M.Kamlar,
J.Vesely,
P.Hobza,
A.Podjarny,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.24 / 2.05 |
| Space group | P 31 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.458, 79.458, 49.418, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.1 / 22.2 |
Other elements in 5liy:
The structure of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Bromine Binding Sites:
The binding sites of Bromine atom in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
(pdb code 5liy). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 10 binding sites of Bromine where determined in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204, PDB code: 5liy:
Jump to Bromine binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
In total 10 binding sites of Bromine where determined in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204, PDB code: 5liy:
Jump to Bromine binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Bromine binding site 1 out of 10 in 5liy
Go back to
Bromine binding site 1 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
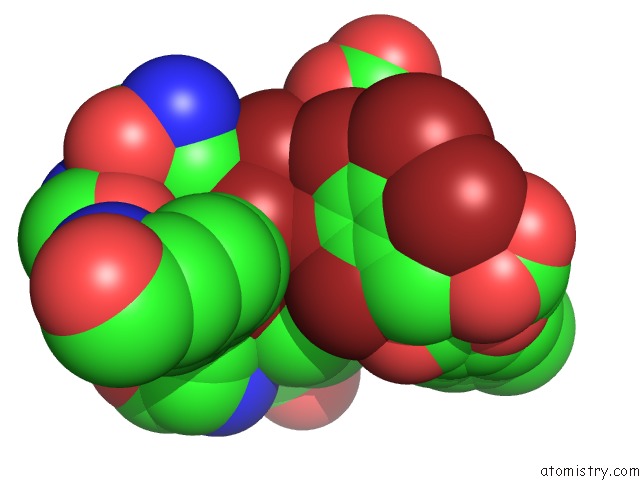
Mono view
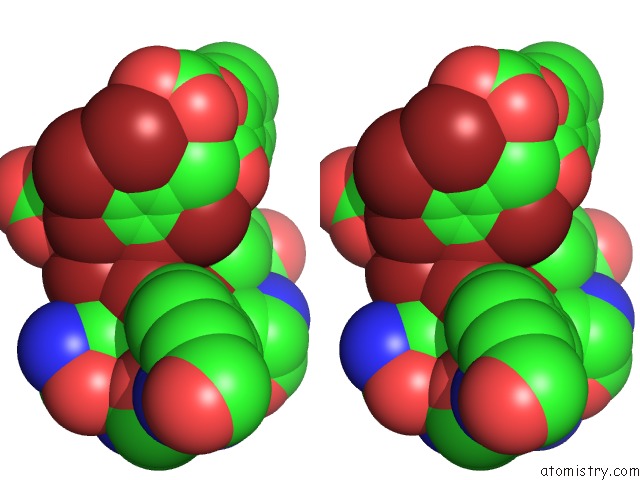
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 2 out of 10 in 5liy
Go back to
Bromine binding site 2 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
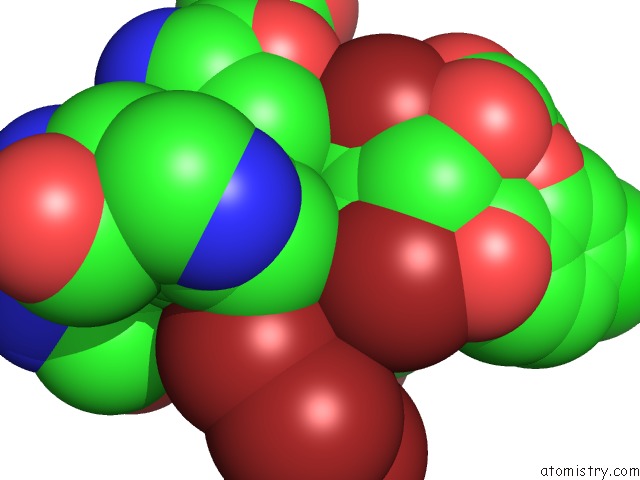
Mono view
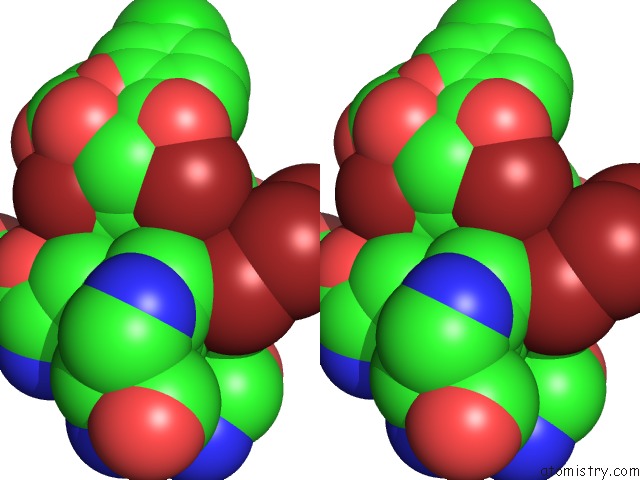
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 3 out of 10 in 5liy
Go back to
Bromine binding site 3 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 4 out of 10 in 5liy
Go back to
Bromine binding site 4 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 4 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 5 out of 10 in 5liy
Go back to
Bromine binding site 5 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
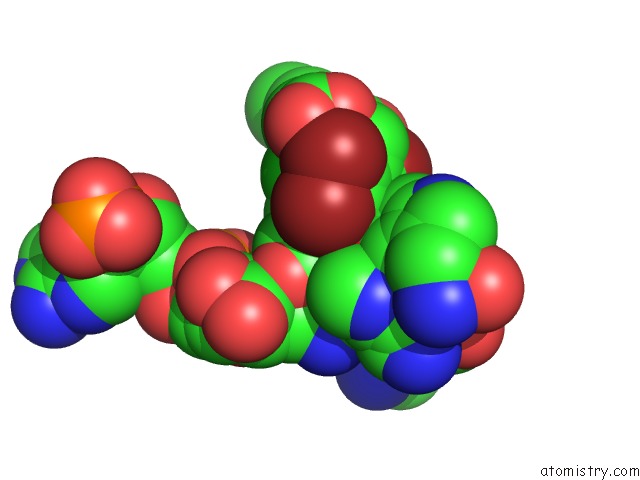
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 5 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 6 out of 10 in 5liy
Go back to
Bromine binding site 6 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
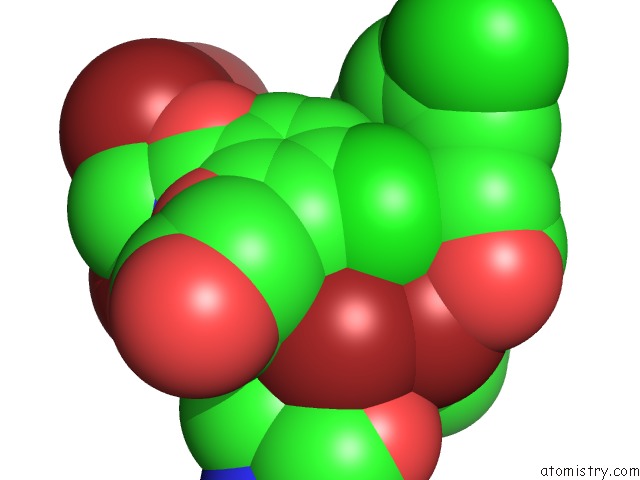
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 6 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 7 out of 10 in 5liy
Go back to
Bromine binding site 7 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
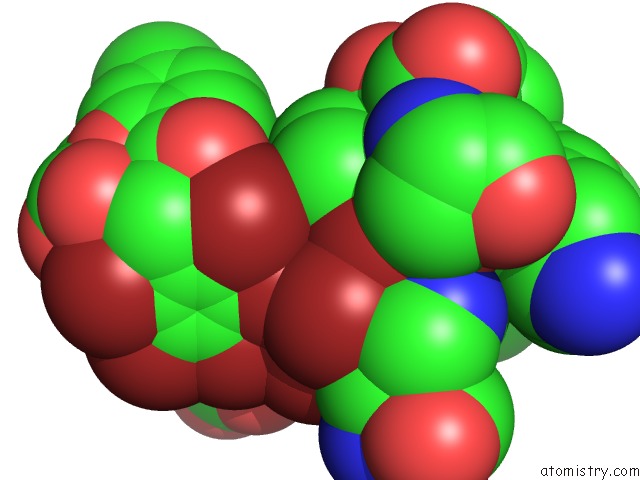
Mono view
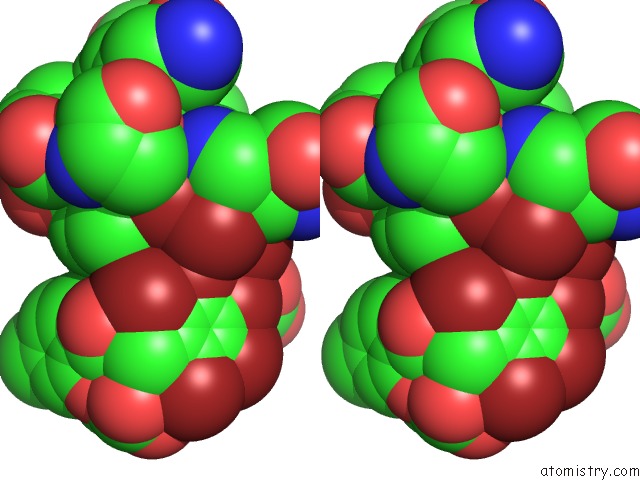
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 7 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 8 out of 10 in 5liy
Go back to
Bromine binding site 8 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
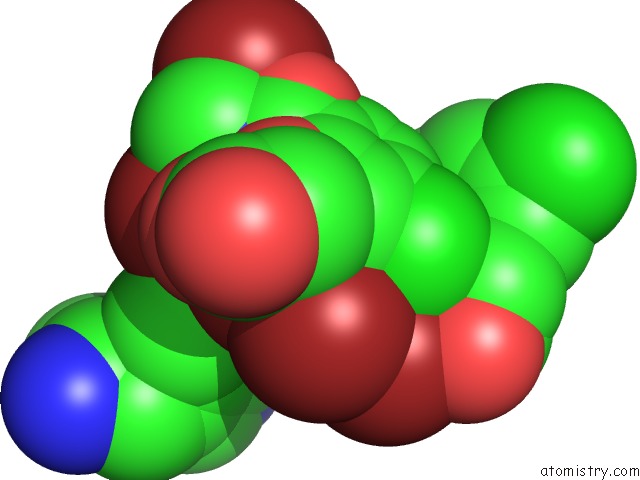
Mono view
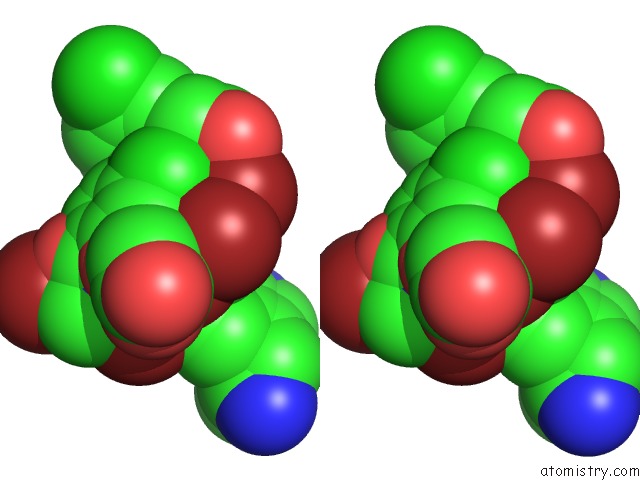
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 8 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 9 out of 10 in 5liy
Go back to
Bromine binding site 9 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 9 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Bromine binding site 10 out of 10 in 5liy
Go back to
Bromine binding site 10 out
of 10 in the Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 10 of Crystal Structure of Human AKR1B10 Complexed with Nadp+ and the Inhibitor MK204 within 5.0Å range:
|
Reference:
A.Cousido-Siah,
F.X.Ruiz,
J.Fanfrlik,
J.Gimenez-Dejoz,
A.Mitschler,
M.Kamlar,
J.Vesely,
H.Ajani,
X.Pares,
J.Farres,
P.Hobza,
A.D.Podjarny.
IDD388 Polyhalogenated Derivatives As Probes For An Improved Structure-Based Selectivity of AKR1B10 Inhibitors. Acs Chem.Biol. V. 11 2693 2016.
ISSN: ESSN 1554-8937
PubMed: 27359042
DOI: 10.1021/ACSCHEMBIO.6B00382
Page generated: Mon Jul 7 08:35:47 2025
ISSN: ESSN 1554-8937
PubMed: 27359042
DOI: 10.1021/ACSCHEMBIO.6B00382
Last articles
F in 7G32F in 7G46
F in 7G3I
F in 7G3Z
F in 7G30
F in 7G3C
F in 7G31
F in 7G2S
F in 7G2Y
F in 7G2V