Bromine »
PDB 5t7a-5v1k »
5u79 »
Bromine in PDB 5u79: Crystal Structure of A Complex Formed Between Merb and Dimethyltin
Enzymatic activity of Crystal Structure of A Complex Formed Between Merb and Dimethyltin
All present enzymatic activity of Crystal Structure of A Complex Formed Between Merb and Dimethyltin:
4.99.1.2;
4.99.1.2;
Protein crystallography data
The structure of Crystal Structure of A Complex Formed Between Merb and Dimethyltin, PDB code: 5u79
was solved by
H.M.Wahba,
M.Stevenson,
A.Mansour,
J.Sygusch,
D.E.Wilcox,
J.G.Omichinski,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 26.06 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 38.585, 89.236, 54.680, 90.00, 98.48, 90.00 |
| R / Rfree (%) | 17 / 20.4 |
Other elements in 5u79:
The structure of Crystal Structure of A Complex Formed Between Merb and Dimethyltin also contains other interesting chemical elements:
| Tin | (Sn) | 2 atoms |
Bromine Binding Sites:
The binding sites of Bromine atom in the Crystal Structure of A Complex Formed Between Merb and Dimethyltin
(pdb code 5u79). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total only one binding site of Bromine was determined in the Crystal Structure of A Complex Formed Between Merb and Dimethyltin, PDB code: 5u79:
In total only one binding site of Bromine was determined in the Crystal Structure of A Complex Formed Between Merb and Dimethyltin, PDB code: 5u79:
Bromine binding site 1 out of 1 in 5u79
Go back to
Bromine binding site 1 out
of 1 in the Crystal Structure of A Complex Formed Between Merb and Dimethyltin
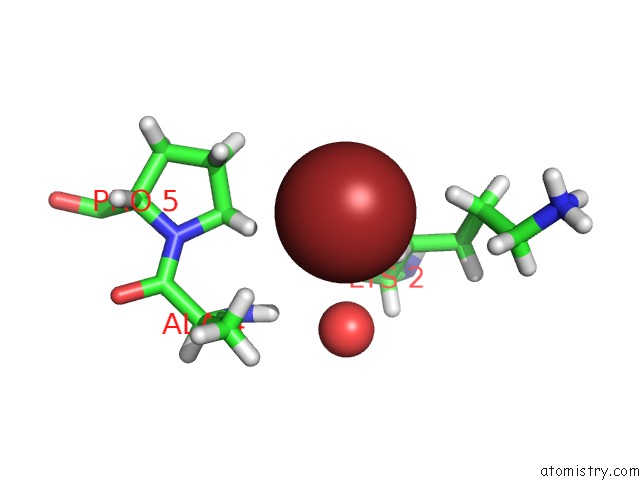
Mono view
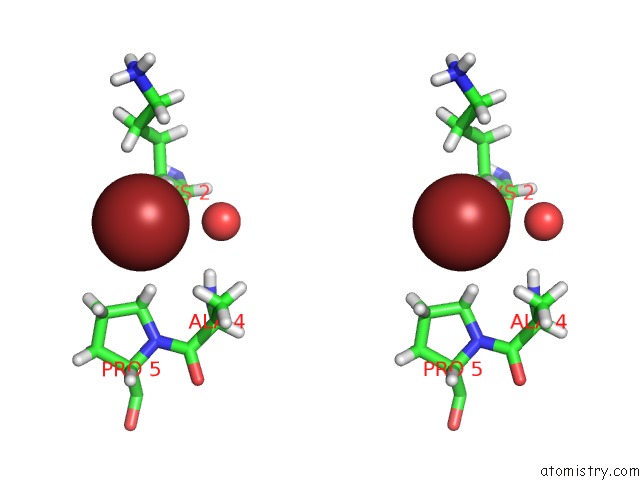
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Crystal Structure of A Complex Formed Between Merb and Dimethyltin within 5.0Å range:
|
Reference:
H.M.Wahba,
M.J.Stevenson,
A.Mansour,
J.Sygusch,
D.E.Wilcox,
J.G.Omichinski.
Structural and Biochemical Characterization of Organotin and Organolead Compounds Binding to the Organomercurial Lyase Merb Provide New Insights Into Its Mechanism of Carbon-Metal Bond Cleavage. J. Am. Chem. Soc. V. 139 910 2017.
ISSN: ESSN 1520-5126
PubMed: 27989130
DOI: 10.1021/JACS.6B11327
Page generated: Thu Jul 11 01:10:10 2024
ISSN: ESSN 1520-5126
PubMed: 27989130
DOI: 10.1021/JACS.6B11327
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF