Bromine »
PDB 5y94-6c2x »
6ada »
Bromine in PDB 6ada: Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide
Protein crystallography data
The structure of Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide, PDB code: 6ada
was solved by
H.-H.Lim,
K.Park,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 33.97 / 3.15 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 232.409, 99.956, 170.811, 90.00, 131.88, 90.00 |
| R / Rfree (%) | 23.1 / 29 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide
(pdb code 6ada). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 4 binding sites of Bromine where determined in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide, PDB code: 6ada:
Jump to Bromine binding site number: 1; 2; 3; 4;
In total 4 binding sites of Bromine where determined in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide, PDB code: 6ada:
Jump to Bromine binding site number: 1; 2; 3; 4;
Bromine binding site 1 out of 4 in 6ada
Go back to
Bromine binding site 1 out
of 4 in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide
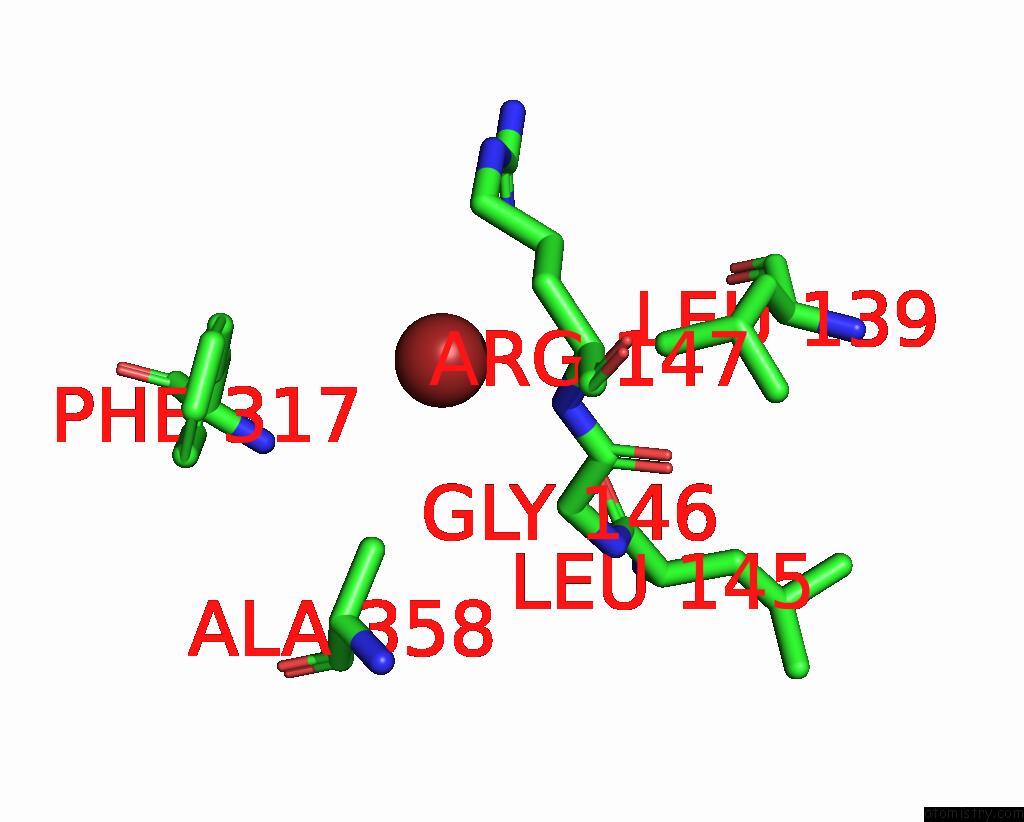
Mono view
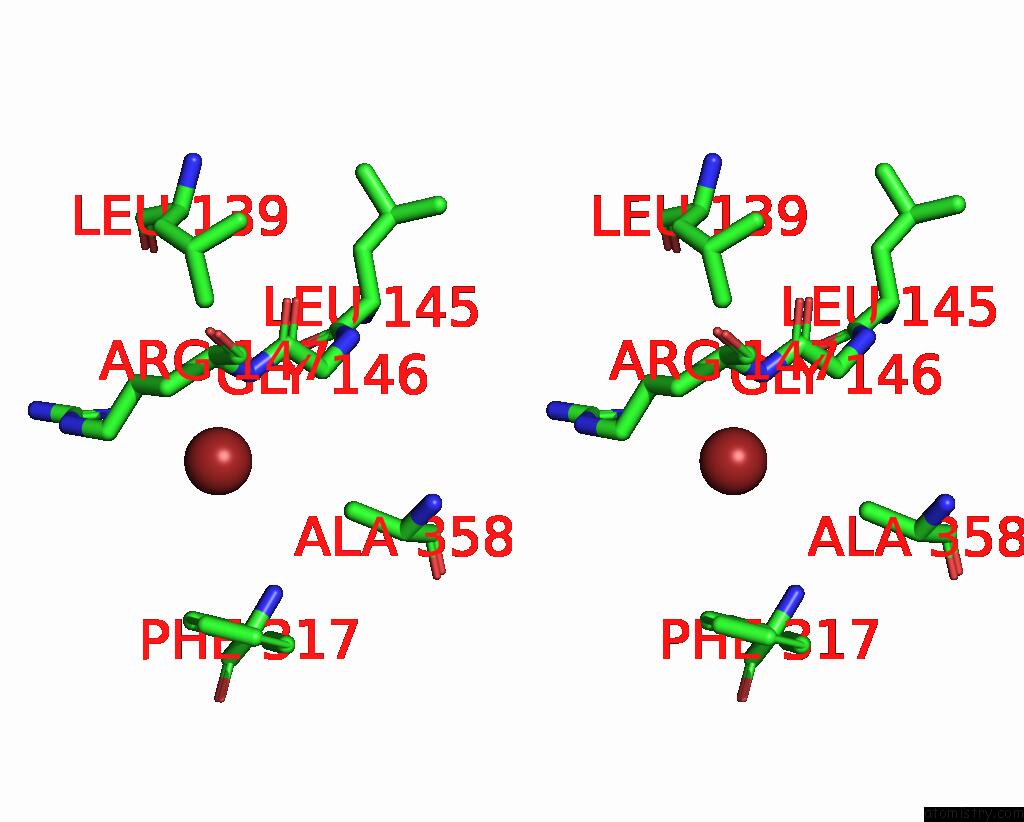
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide within 5.0Å range:
|
Bromine binding site 2 out of 4 in 6ada
Go back to
Bromine binding site 2 out
of 4 in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide
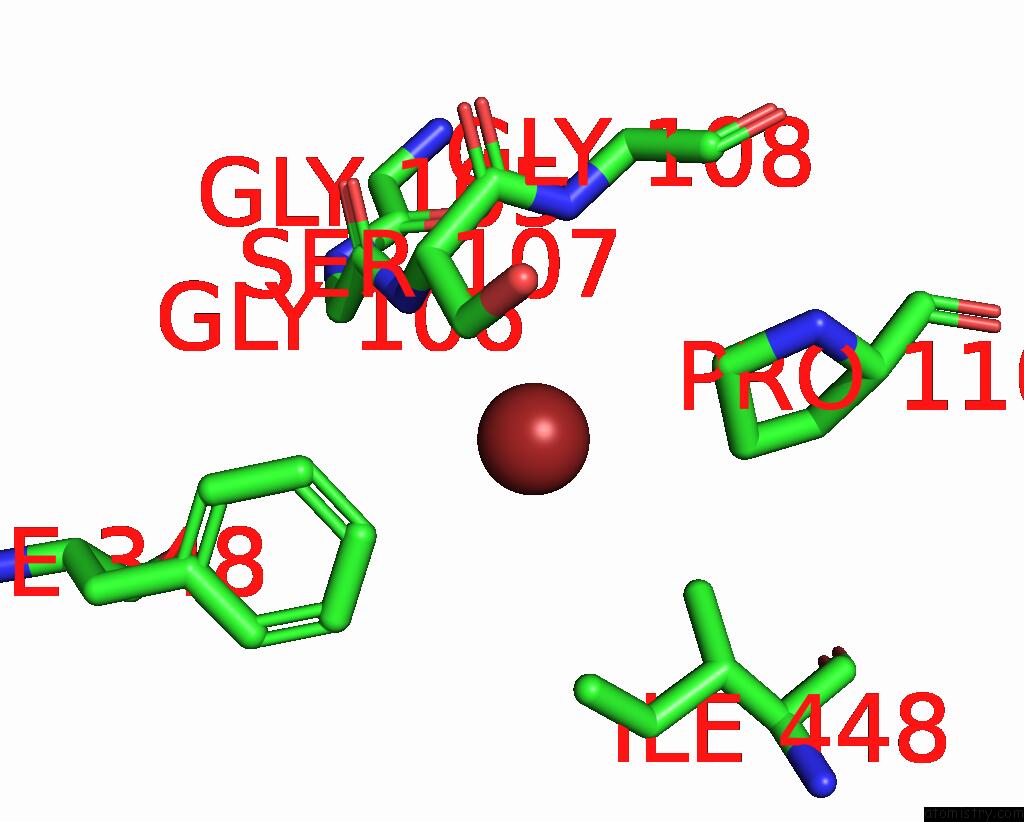
Mono view
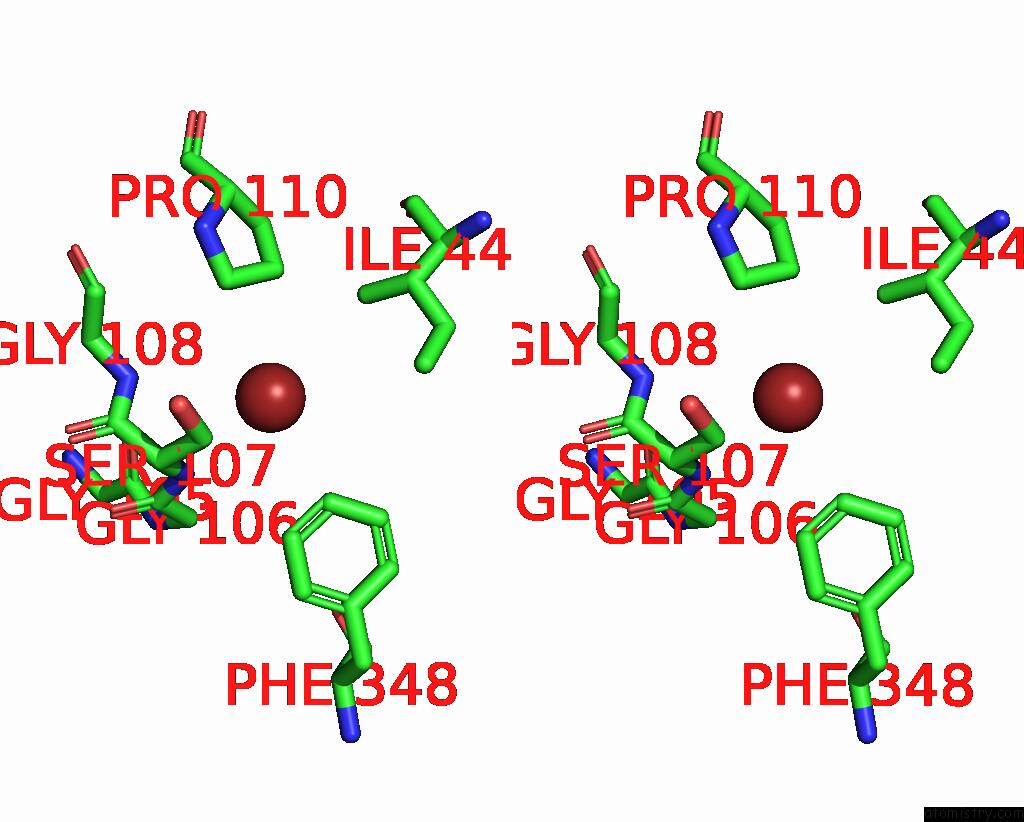
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide within 5.0Å range:
|
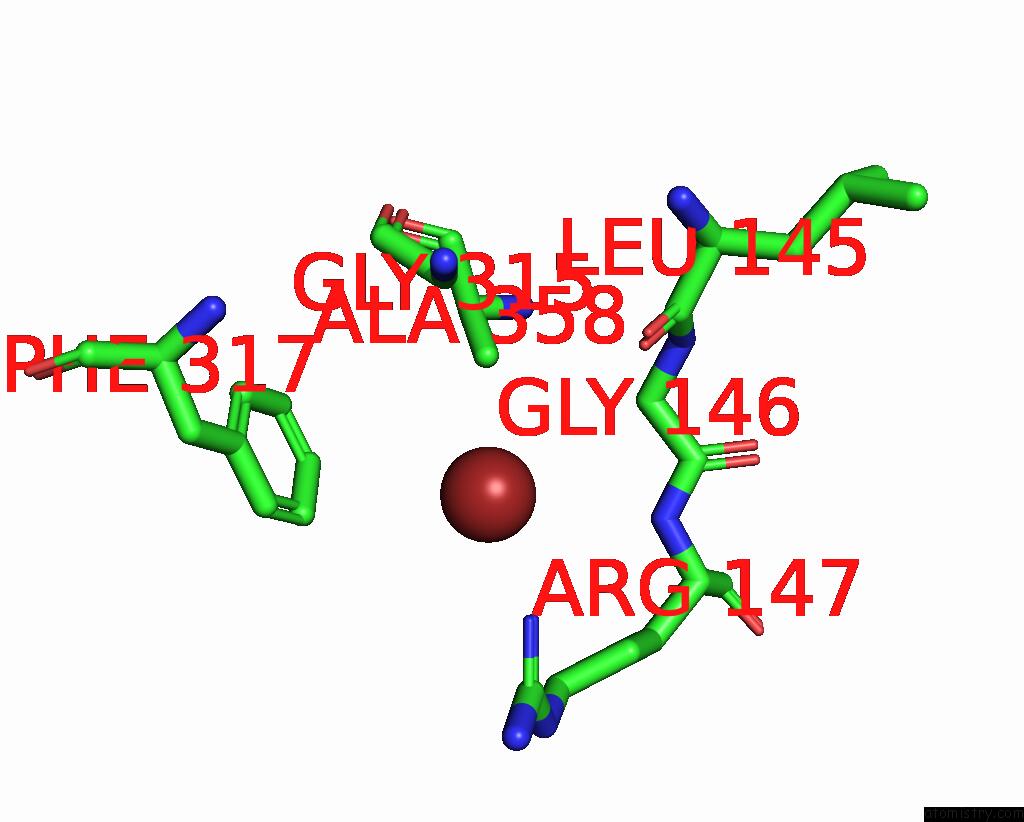
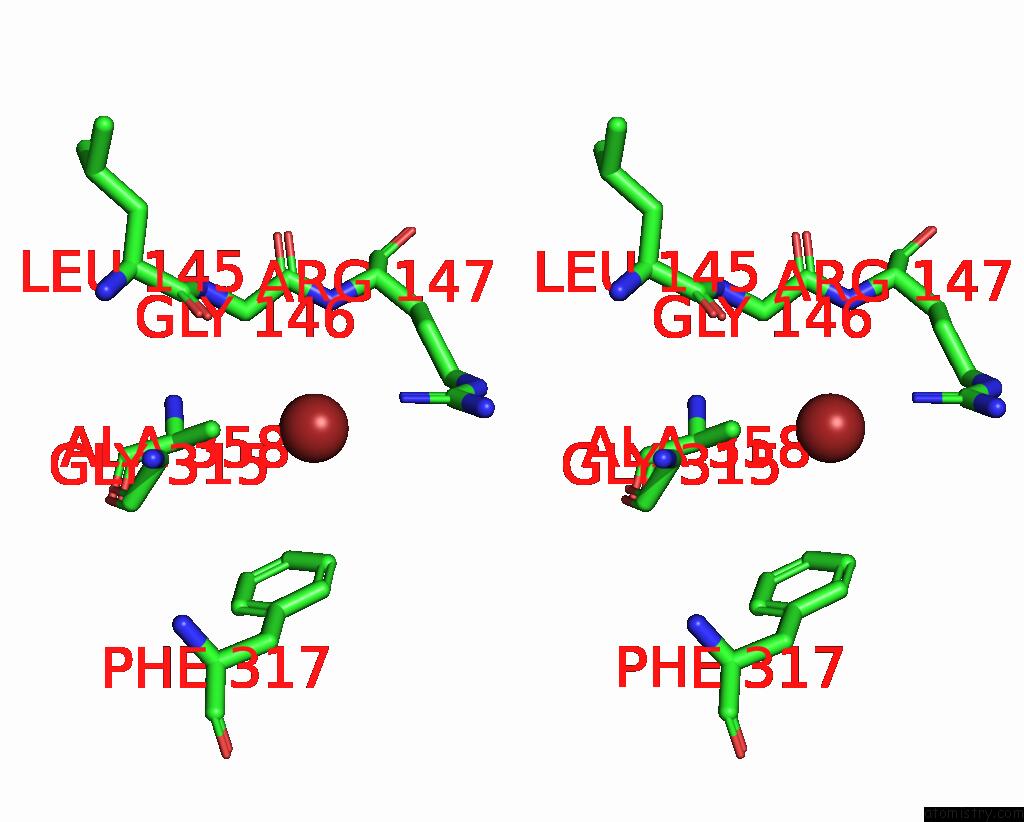
Bromine binding site 3 out of 4 in 6ada
Go back to
Bromine binding site 3 out
of 4 in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide within 5.0Å range:
|
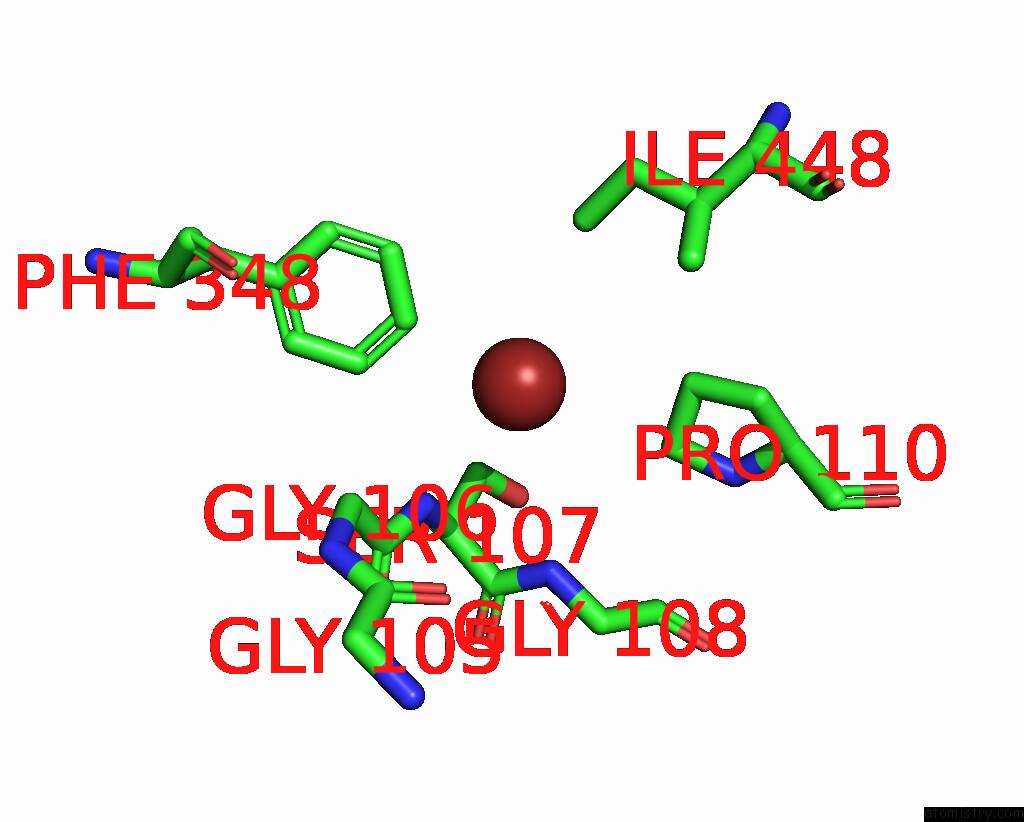
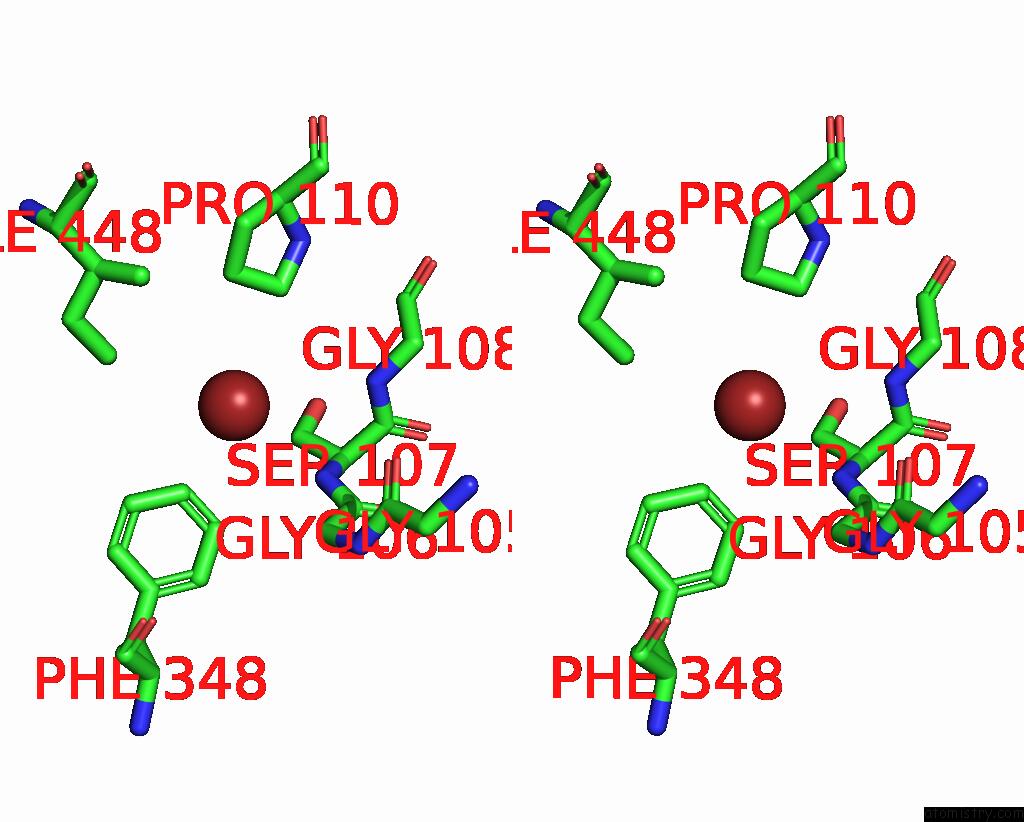
Bromine binding site 4 out of 4 in 6ada
Go back to
Bromine binding site 4 out
of 4 in the Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 4 of Crystal Structure of the E148D Mutant Clc-EC1 in 200MM Bromide within 5.0Å range:
|
Reference:
K.Park,
B.C.Lee,
H.H.Lim.
Mutation of External Glutamate Residue Reveals A New Intermediate Transport State and Anion Binding Site in A Clc Cl-/H+Antiporter. Proc.Natl.Acad.Sci.Usa V. 116 17345 2019.
ISSN: ESSN 1091-6490
PubMed: 31409705
DOI: 10.1073/PNAS.1901822116
Page generated: Mon Jul 7 09:30:28 2025
ISSN: ESSN 1091-6490
PubMed: 31409705
DOI: 10.1073/PNAS.1901822116
Last articles
Ca in 1GZACa in 1GZ9
Ca in 1GXR
Ca in 1GQM
Ca in 1GX2
Ca in 1GXD
Ca in 1GXO
Ca in 1GWU
Ca in 1GWT
Ca in 1GWO