Bromine »
PDB 6yod-7akh »
6yvz »
Bromine in PDB 6yvz: Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367
Enzymatic activity of Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367
All present enzymatic activity of Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367:
1.14.11.29;
1.14.11.29;
Protein crystallography data
The structure of Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367, PDB code: 6yvz
was solved by
R.Chowdhury,
J.L.Sorensen,
C.J.Schofield,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 36.15 / 1.91 |
| Space group | P 63 |
| Cell size a, b, c (Å), α, β, γ (°) | 109.88, 109.88, 39.08, 90, 90, 120 |
| R / Rfree (%) | 16.7 / 20.4 |
Other elements in 6yvz:
The structure of Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367 also contains other interesting chemical elements:
| Manganese | (Mn) | 1 atom |
Bromine Binding Sites:
The binding sites of Bromine atom in the Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367
(pdb code 6yvz). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total only one binding site of Bromine was determined in the Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367, PDB code: 6yvz:
In total only one binding site of Bromine was determined in the Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367, PDB code: 6yvz:
Bromine binding site 1 out of 1 in 6yvz
Go back to
Bromine binding site 1 out
of 1 in the Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367
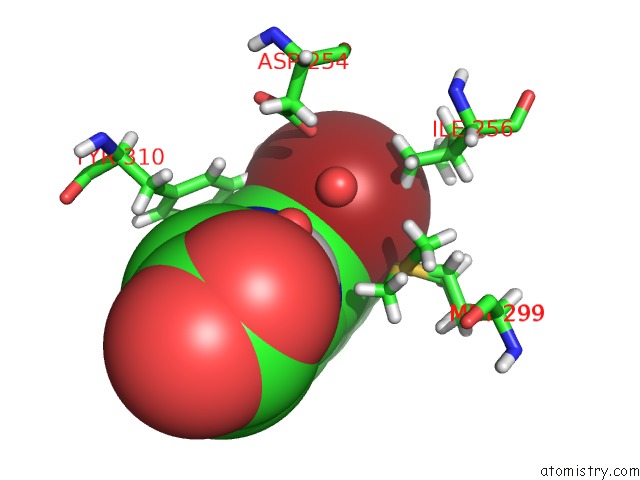
Mono view
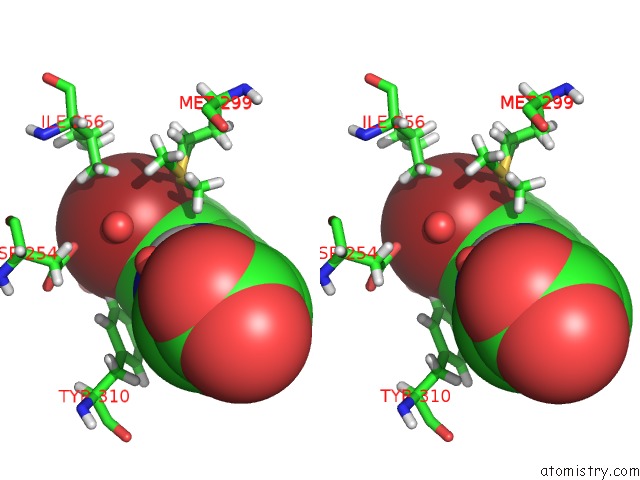
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Hif Prolyl Hydroxylase 2 (PHD2/ EGLN1) in Complex with Bicyclic Jls- 367 within 5.0Å range:
|
Reference:
R.Chowdhury,
M.I.Abboud,
T.E.Mcallister,
B.Banerji,
B.Bhushan,
J.L.Sorensen,
A.Kawamura,
C.J.Schofield.
Use of Cyclic Peptides to Induce Crystallization: Case Study with Prolyl Hydroxylase Domain 2. Sci Rep V. 10 21964 2020.
ISSN: ESSN 2045-2322
PubMed: 33319810
DOI: 10.1038/S41598-020-76307-8
Page generated: Mon Jul 7 10:47:22 2025
ISSN: ESSN 2045-2322
PubMed: 33319810
DOI: 10.1038/S41598-020-76307-8
Last articles
Cl in 3TXFCl in 3TXE
Cl in 3TXD
Cl in 3TXB
Cl in 3TW9
Cl in 3TX8
Cl in 3TX3
Cl in 3TX6
Cl in 3TWB
Cl in 3TW6