Bromine »
PDB 4kvh-4my6 »
4mk1 »
Bromine in PDB 4mk1: 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
Protein crystallography data
The structure of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease, PDB code: 4mk1
was solved by
J.D.Bauman,
D.Patel,
K.Das,
E.Arnold,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.32 / 1.85 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.932, 101.655, 66.212, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.6 / 19.8 |
Other elements in 4mk1:
The structure of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease also contains other interesting chemical elements:
| Manganese | (Mn) | 4 atoms |
Bromine Binding Sites:
The binding sites of Bromine atom in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
(pdb code 4mk1). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 3 binding sites of Bromine where determined in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease, PDB code: 4mk1:
Jump to Bromine binding site number: 1; 2; 3;
In total 3 binding sites of Bromine where determined in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease, PDB code: 4mk1:
Jump to Bromine binding site number: 1; 2; 3;
Bromine binding site 1 out of 3 in 4mk1
Go back to
Bromine binding site 1 out
of 3 in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
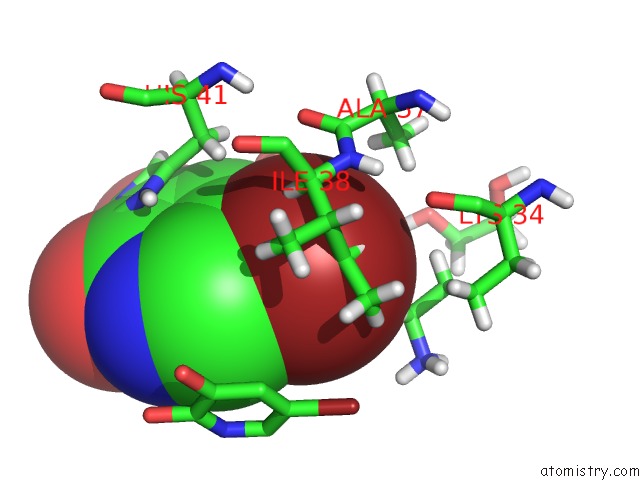
Mono view
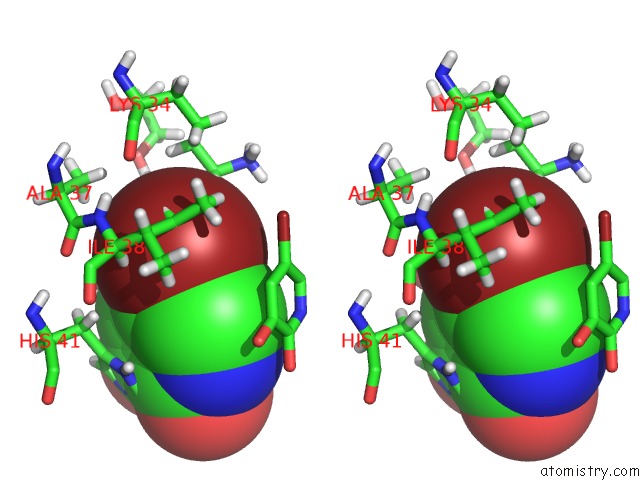
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease within 5.0Å range:
|
Bromine binding site 2 out of 3 in 4mk1
Go back to
Bromine binding site 2 out
of 3 in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
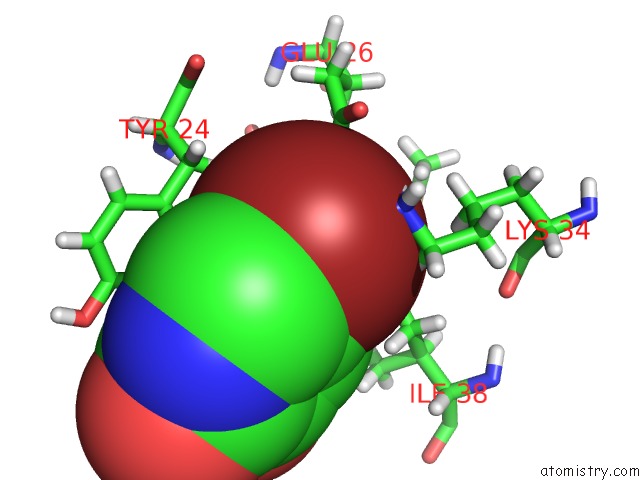
Mono view
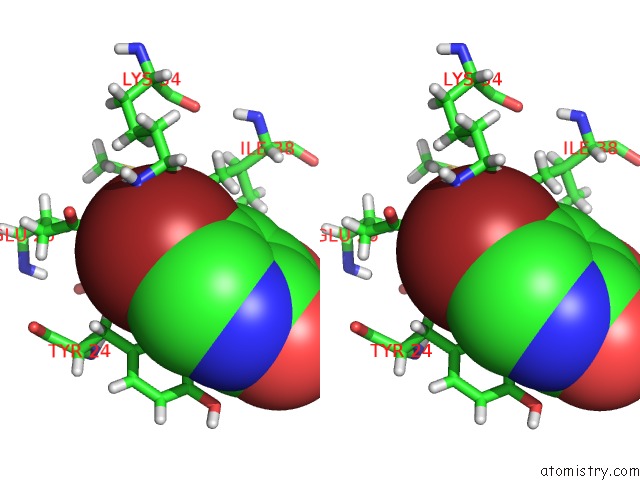
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease within 5.0Å range:
|
Bromine binding site 3 out of 3 in 4mk1
Go back to
Bromine binding site 3 out
of 3 in the 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease
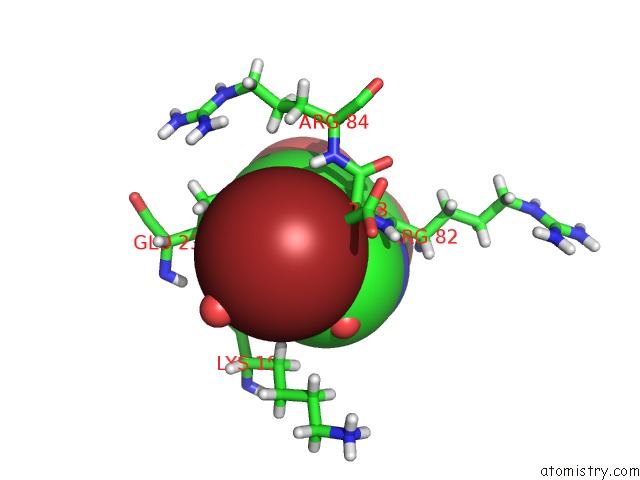
Mono view
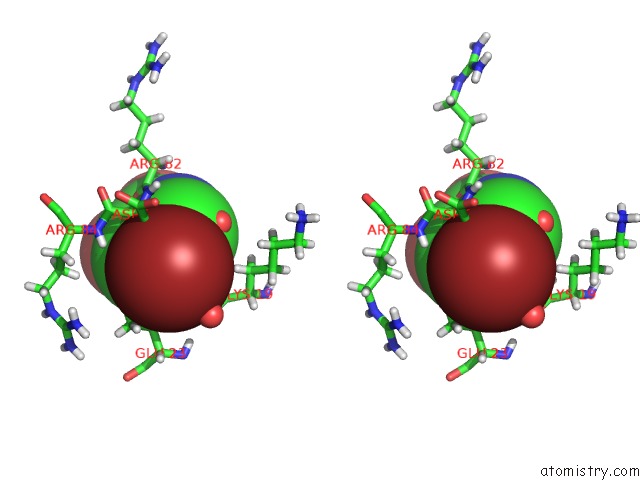
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 3 of 5-Bromopyridine-2,3-Diol Bound to Influenza 2009 PH1N1 Endonuclease within 5.0Å range:
|
Reference:
J.D.Bauman,
D.Patel,
S.F.Baker,
R.S.Vijayan,
A.Xiang,
A.K.Parhi,
L.Martinez-Sobrido,
E.J.Lavoie,
K.Das,
E.Arnold.
Crystallographic Fragment Screening and Structure-Based Optimization Yields A New Class of Influenza Endonuclease Inhibitors. Acs Chem.Biol. V. 8 2501 2013.
ISSN: ISSN 1554-8929
PubMed: 23978130
DOI: 10.1021/CB400400J
Page generated: Mon Jul 7 07:06:18 2025
ISSN: ISSN 1554-8929
PubMed: 23978130
DOI: 10.1021/CB400400J
Last articles
K in 5AVUK in 5AVW
K in 5AVV
K in 5AVR
K in 5AVQ
K in 5AVT
K in 5AVS
K in 5AOH
K in 5AOP
K in 5AMM