Bromine »
PDB 4x6i-4z8b »
4x6i »
Bromine in PDB 4x6i: Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors.
Enzymatic activity of Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors.
All present enzymatic activity of Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors.:
3.4.22.38;
3.4.22.38;
Protein crystallography data
The structure of Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors., PDB code: 4x6i
was solved by
J.Borisek,
B.Mohar,
M.Vizovisek,
P.Sosnowski,
D.Turk,
B.Turk,
M.Novic,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.28 / 1.87 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 36.459, 54.450, 87.870, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.1 / 23.7 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors.
(pdb code 4x6i). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total only one binding site of Bromine was determined in the Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors., PDB code: 4x6i:
In total only one binding site of Bromine was determined in the Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors., PDB code: 4x6i:
Bromine binding site 1 out of 1 in 4x6i
Go back to
Bromine binding site 1 out
of 1 in the Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors.
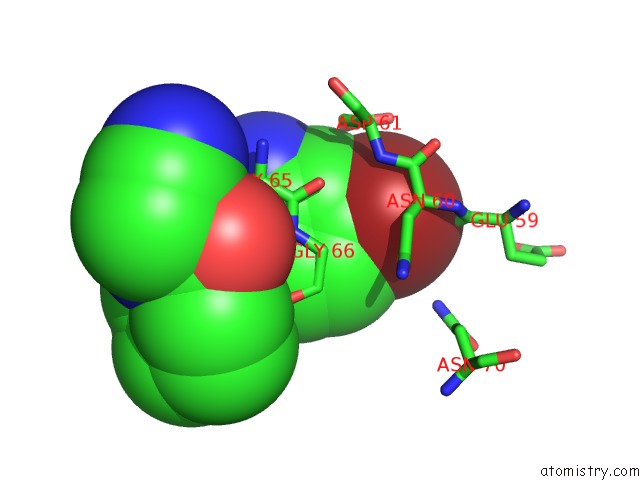
Mono view
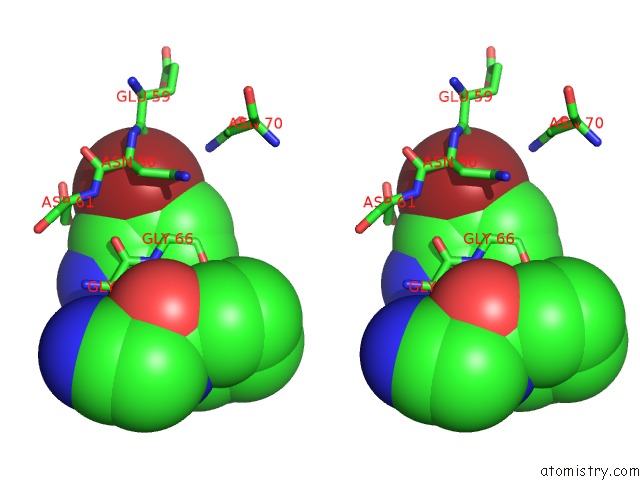
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Development of N-(Functionalized Benzoyl)-Homocycloleucyl- Glycinonitriles As Potent Cathepsin K Inhibitors. within 5.0Å range:
|
Reference:
J.Borisek,
M.Vizovisek,
P.Sosnowski,
B.Turk,
D.Turk,
B.Mohar,
M.Novic.
Development of N-(Functionalized Benzoyl)-Homocycloleucyl-Glycinonitriles As Potent Cathepsin K Inhibitors. J.Med.Chem. V. 58 6928 2015.
ISSN: ISSN 0022-2623
PubMed: 26280490
DOI: 10.1021/ACS.JMEDCHEM.5B00746
Page generated: Mon Jul 7 07:48:00 2025
ISSN: ISSN 0022-2623
PubMed: 26280490
DOI: 10.1021/ACS.JMEDCHEM.5B00746
Last articles
V in 1IDQV in 1B8J
V in 1DFL
V in 1BO6
V in 1C4G
U in 6BJY
U in 7B2W
U in 3PU4
U in 5VCR
U in 4ILD