Bromine »
PDB 5q0w-5s9t »
5rc4 »
Bromine in PDB 5rc4: Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A
Enzymatic activity of Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A
All present enzymatic activity of Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A:
3.4.23.22;
3.4.23.22;
Protein crystallography data
The structure of Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A, PDB code: 5rc4
was solved by
M.S.Weiss,
J.Wollenhaupt,
A.Metz,
T.Barthel,
G.M.A.Lima,
A.Heine,
U.Mueller,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.74 / 1.00 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 45.238, 72.821, 52.411, 90.00, 109.25, 90.00 |
| R / Rfree (%) | 15.9 / 16 |
Bromine Binding Sites:
The binding sites of Bromine atom in the Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A
(pdb code 5rc4). This binding sites where shown within
5.0 Angstroms radius around Bromine atom.
In total 2 binding sites of Bromine where determined in the Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A, PDB code: 5rc4:
Jump to Bromine binding site number: 1; 2;
In total 2 binding sites of Bromine where determined in the Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A, PDB code: 5rc4:
Jump to Bromine binding site number: 1; 2;
Bromine binding site 1 out of 2 in 5rc4
Go back to
Bromine binding site 1 out
of 2 in the Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A

Mono view
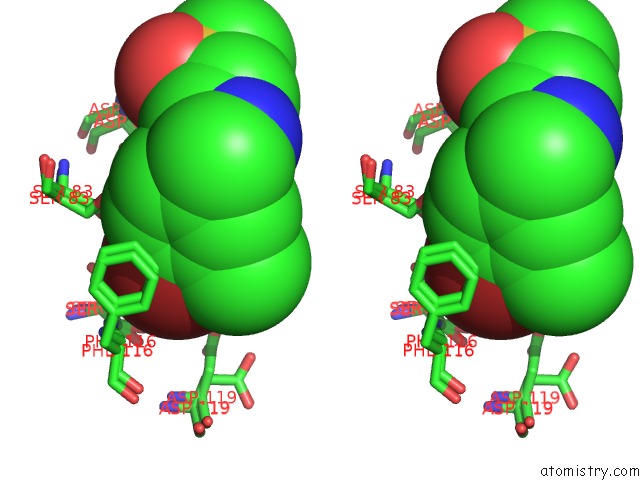
Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 1 of Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A within 5.0Å range:
|
Bromine binding site 2 out of 2 in 5rc4
Go back to
Bromine binding site 2 out
of 2 in the Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A
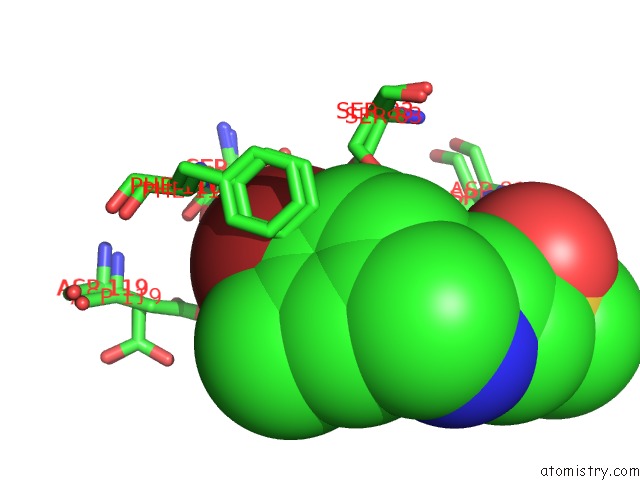
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Bromine with other atoms in the Br binding
site number 2 of Pandda Analysis Group Deposition -- Endothiapepsin Changed State Model For Fragment F2X-Entry Library F04A within 5.0Å range:
|
Reference:
J.Wollenhaupt,
A.Metz,
T.Barthel,
G.M.A.Lima,
A.Heine,
U.Mueller,
G.Klebe,
M.S.Weiss.
F2X-Universal and F2X-Entry: Structurally Diverse Compound Libraries For Crystallographic Fragment Screening. Structure 2020.
ISSN: ISSN 0969-2126
PubMed: 32413289
DOI: 10.1016/J.STR.2020.04.019
Page generated: Mon Jul 7 09:03:31 2025
ISSN: ISSN 0969-2126
PubMed: 32413289
DOI: 10.1016/J.STR.2020.04.019
Last articles
V in 1UZIV in 1YV3
V in 1VOM
V in 1VNI
V in 1VNH
V in 1VNG
V in 1VNF
V in 1VNE
V in 1VNC
V in 1VFZ